
Guardant Health
@GuardantHealth
Conquering Cancer With Data 🩸
Last week, our co-CEO AmirAli Talasaz took the stage at #AspenIdeasHealth on the New Frontiers in Cancer panel, a timely discussion on how breakthroughs in blood-based screening, precision medicine, and AI are reshaping the future of care. From expanding access to early…



At #AspenIdeasHealth, our co-CEO AmirAli Talasaz sat down with @DrJohnTorres to discuss a critical gap in cancer care: screening technologies are advancing, but access isn’t keeping pace. Our FDA-approved Shield™ blood test has the potential to expand access to early detection…
Today, our co-CEO AmirAli Talasaz joins the New Frontiers in Cancer panel at #AspenIdeasHealth to discuss how innovation in blood-based screening, AI, and precision medicine is reshaping the fight against cancer. Tune in via @AspenIdeas to join the conversation!

Featured in @nytimes: Our Guardant360® CDx test is helping revolutionize #BreastCancer treatment by detecting drug resistance before it shows up on scans—enabling timely treatment changes and improving patient outcomes. nytimes.com/2025/06/01/hea…
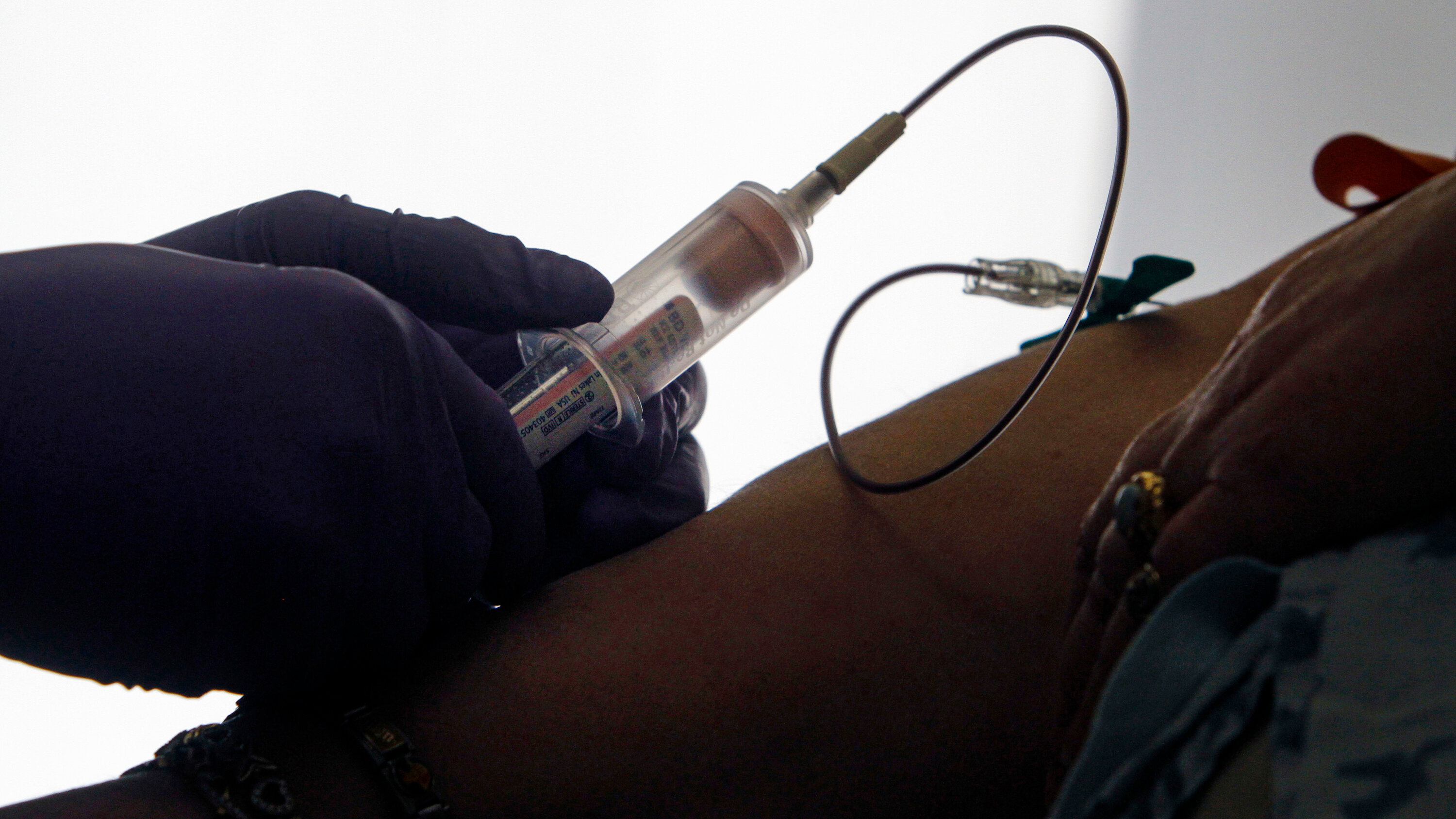
Today we announced that the first patient has been enrolled in the @theNCI’s Vanguard Study, evaluating our Shield™ MCD test. The four-year study will assess the feasibility of using blood-based tests like Shield to detect multiple cancers in individuals without symptoms.
AI-Powered Oncology and Genomic Testing Converge with VieCure and Guardant Healthcare Partnership @VieCure's partnership with @GuardantHealth consolidates liquid and tissue-based diagnostics #cancertesting #EHR #liquidbiopsy hubs.li/Q03wXBwq0
Our precision oncology tests are now integrated into the VieCure Halo Intelligence platform, giving oncologists streamlined access to critical insights and enabling community oncology practices to make faster, more personalized treatment decisions for their patients—without…

Join us for a live #Webinar on the power of epigenomic biomarkers to improve patient selection and better predict treatment response in oncology clinical trials. While genomic alterations provide critical insight into tumor biology, they often don’t tell the full story.…

Exciting news! Our Shield™ blood test for #ColorectalCancer screening has been recognized on @FastCompany's 2025 World Changing Ideas list! We're thrilled to be part of this amazing collection of ideas driving meaningful change in the world. Check out the full list here:…

Today, we’re proud to share that @ESMO_Open has published results from the LIBERATE study, highlighting Guardant Reveal’s performance in detecting minimal residual disease in early-stage #BreastCancer.
We're honored to announce that our Shield multi-cancer detection (MCD) test has been granted Breakthrough Device designation from the @US_FDA.
We’re proud to share that the @NCCN has included our Shield™ blood test in its updated #ColorectalCancer screening guidelines. Marking a pivotal step forward in expanding #PatientAccess to an innovative, blood-based screening option.
Results from the SERENA-6 Phase III trial, presented at #ASCO25 and published in @NEJM, show how Guardant360® CDx can catch emerging ESR1 mutations in advanced breast cancer—giving clinicians a chance to adjust treatment before cancer progresses.
Presented at #ASCO25: In patients with advanced breast cancer, switching to camizestrant with a CDK4/6 inhibitor after ESR1-mutation detection (and before disease progression) led to significantly longer progression-free survival. Full SERENA-6 phase 3 trial results:…
Today we announced results from the Phase III SERENA-6 trial, demonstrating the clinical value of our Guardant360® CDx test in detecting and treating emerging resistance in advanced #BreastCancer ahead of radiological progression. Study findings were presented at #ASCO25 and…

The future of precision oncology is here. Join us at #ASCO25, booth #25077, to discover the pivotal role of liquid biopsy in transforming cancer care and improving patient outcomes.



At #ASCO25 yesterday, we shared results from the largest study to date evaluating ctDNA in #ColonCancer, highlighting the power of our Guardant Reveal™ test to inform critical treatment decisions.
NHS England's achievement in becoming the first healthcare system in the world to bring liquid biopsy into routine cancer care is a significant milestone and we are honored to have worked on the NHS pilot study, in collaboration with @royalmarsdenNHS and @NHSEngland, which…
Thousands of patients with cancer will benefit from a new blood test which can speed up access to targeted therapy. That's people like Rebeca, who, after being diagnosed with lung cancer, had a liquid biopsy and was able to start treatment faster. england.nhs.uk/2025/05/nhs-fi…
Join us at #ASCO25 for an exclusive event showcasing how our Guardant Infinity™ platform is propelling patient-centered care into the future. Discover how we’re unlocking new possibilities across the patient journey and redefining precision oncology through innovation and…
