Luke Allsopp
@luke_allsopp
Lecturer @ImperialNHLI at @imperialcollege London #Microbiology #Bacteriology #Scientist #Biofilm #Bacteria #Nature All views my own RT not endorsements
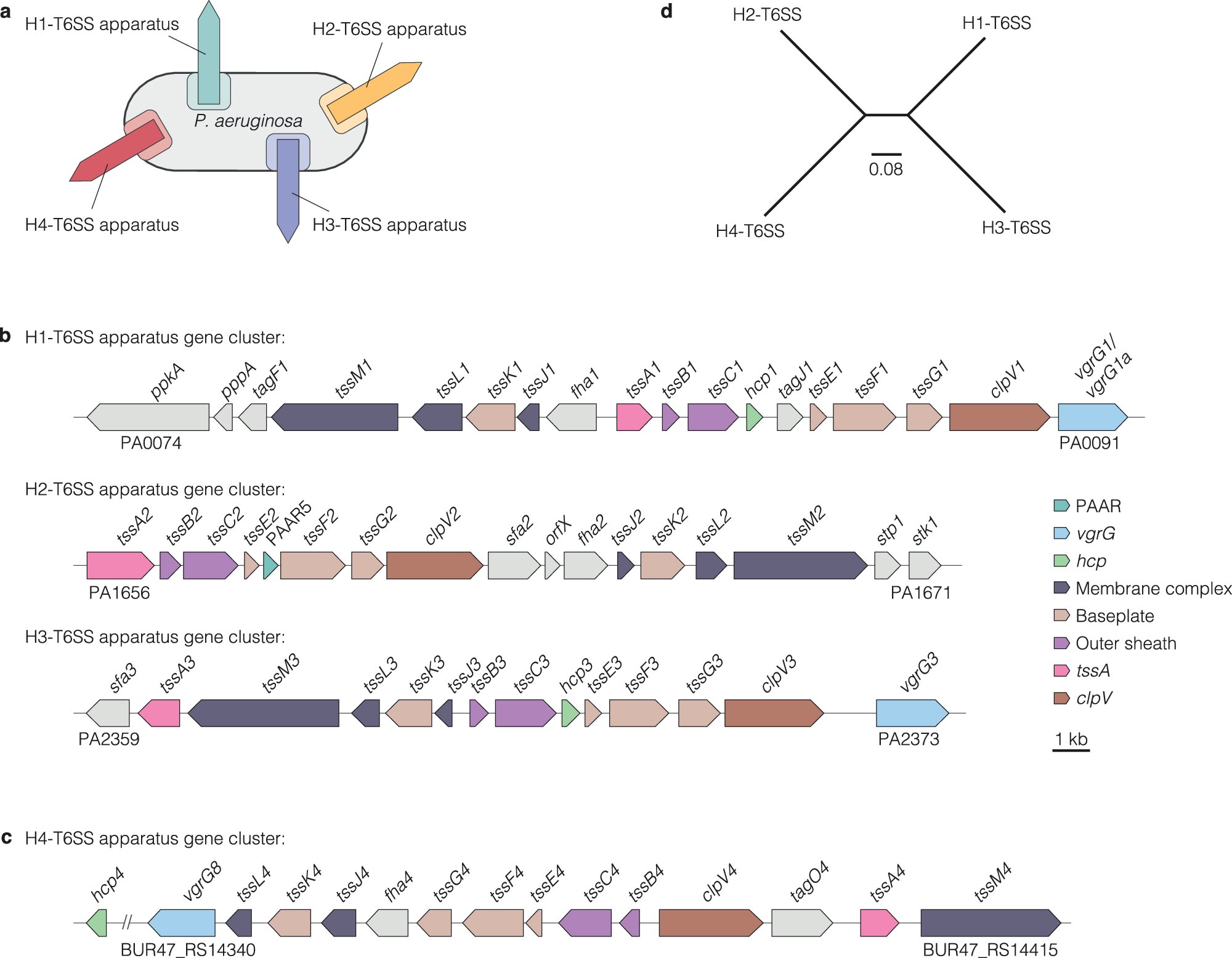
Latest out from the lab. Crazy amounts of #diversity in #T6SS #effectors #pseudomonas out now in @NatureComms @MPI_EvolBio @ImperialNHLI imperial.ac.uk/news/260613/ba… evolbio.mpg.de/3804447/same-b… nature.com/articles/s4146…
🎉 Congratulations to Sue Francis, Gill Martin, Hime Gashaw & Mandy Hipwell from NHLI on winning the Provost’s Award for Excellence in Health & Safety! Their leadership during the VPD building move set a new standard for collaborative safety planning.👏 🔗imperial.ac.uk/news/265833/sa…
Harriet Ellis, a postdoc in @luke_allsopp's Group, is uncovering how Pseudomonas aeruginosa – a key bacterial threat in cystic fibrosis – adapts & causes infection. 💛🦠 #CFweek Discover how her work could help fight lung infections 👇 blogs.imperial.ac.uk/nhli/2025/06/0…
Still time to apply for this PhD position with our team!
Fully funded PhD position available to join our team! We will focus on untangling the mechanisms used by antibiotic resistant E.coli to colonise the host gut. Highly interdisciplinary project with Karrera Djoko and @BanzhafLab. Please share findaphd.com/phds/project/m…
Interested in host-pathogen interactions, secreted effector proteins, and unusual microbes that lead amazing lifestyles? We are recruiting to two positions - a PDRA (48 months) and a Technician (24 months)!
Looking for a Research Project Manager with interest in #CF and #infection to work in a multidisciplinary team based out of @ImperialNHLI @ImperialMed funding from @lifearc1 and @cftrust imperial.ac.uk/jobs/search-jo…
🔬 Did you know that since 2013 we’ve funded over 180 early career researchers, ensuring the brightest and best young scientists have the opportunity to work in CF research? ➡️🧵 #cysticfibrosis #earlycareerresearchers
Formate-specific chemoreceptors and their evolution in bacteria. Very grateful to be part of this multidisciplinary and international research team @KrellLaboratory @Eli_Monteagudo @zhulinlab @gavirius. @BlastMeetings @CSICdivulga @SEMicrobiologia pnas.org/doi/10.1073/pn…
A deep dive into #T6SS effector diversity in #pseudomonas out now in @NatureComms lead by Antonia Habich from the Unterweger Lab. Great science and great collaboration. @MPI_EvolBio @ImperialNHLI nature.com/articles/s4146…

Our latest study shows that the anti-biofilm peptide DJK-5 makes Staphylococcus aureus vulnerable to colistin in co-biofilms with Pseudomonas aeruginosa. A promising step in the fight against chronic co-infections! #AMR #Biofilms nature.com/articles/s4152…
New in JB: Underlying similarity in bacterial adhesins from @MRC_LMB @tbharat_lab. journals.asm.org/doi/10.1128/jb… @ASMicrobiology @JBacteriology
Planning your conference for 2025? Are you into toxins and secretion systems? Registration for ETOX 2025 opens tomorrow 6th until January 31st: etox-meetings.org. It will be amazing, check out our invited speakers 🤩. Go and prepare your abstract and we meet you in Spain!!
I wrote about what you can do to Live Forever in @Telegraph yesterday. Find out what I found out! telegraph.co.uk/health-fitness…
Hearing about NHIR fellowships from @chloebloom @ImperialNHLI Brilliant insights!

Great talk from @DrRBarry at the @ImperialNHLI ECR #grants and #fellowship day!

Fantastic #ECR #Grants and #Fellowship day at @ImperialNHLI thanks to all attendees, organisers, presenters and @ImperialPFDC

JB Editor's Choice: Hourigan, Ross et al. use genomic approaches to identify new leaderless bacterocins in Actinomycetota, and provide experimental evidence for their antimicrobial activity. Such bacteriocins could represent new antimicrobials. @ASMicrobiology @JBacteriology
🚨 Postdoc Opportunity 🚨 The Ronneau Lab is hiring a postdoctoral researcher to join our team in 2025! If you're passionate about microbiology and stress responses in pathogenic bacteria, we want to hear from you! 📍Location: Rennes, France ronneaulab.com
New in JB: The production of alginate in CF isolates of P. aeruginosa is driven by the MucA22 mutation. Sommerfield, Darwin et al. show that the Prc protease targets MucA22 (but not the WT protein). journals.asm.org/doi/10.1128/jb… @ASMicrobiology @JBacteriology
Now out in NSMB for those who missed our biorxiv back in 2023.. rdcu.be/dZSPQ
The missing piece in the bacterial #ESCRT-III story.. #Vipp1 forms dynamic spiral filaments on membrane! Spirals are springs that drive 3D ring formation in the spiral centre! 😎😵💫 Wonderful collaboration with @Colom_D @RouxLab… 1/2 biorxiv.org/content/10.110…