
Christopher Kelly
@ckellzchem
Principal Scientist @ JnJ and Lecturer @ Penn. Lover of old school synthesis, punk rock, astronomy/space, gardening/succulents, and Camaros. Views are my own.
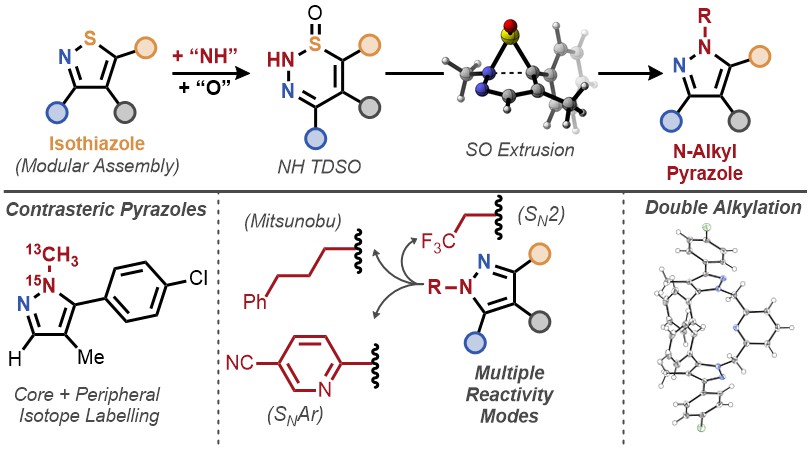
Super proud of how strong @JNJInnovMed collab w/ @LevinChem and @group_levin has become! Our 1st foray for our collab on skeletal edits yielded an cool approach to the synthesis of N-Alkyl Pyrazoles from a non-intuitive SM, isothiazoles! Truly an honor to have been involved in…
The final version of our paper is available, and open access! If you are interested in radical decarboxylative approaches or small rings, please have a look. @snsf_ch @KatayevL @pushingarrows @DCBPunibern
Our Iron-photocatalysis approach to small-ring transfer is now live in its final form @angew_chem! Great collaboration with Julian West @pushingarrows. Congratulations to everyone involved 🚀 @DCBPunibern @SwissChemistry #chemistry Check it out here 👉 onlinelibrary.wiley.com/doi/10.1002/an…
A work many years in the making out today in @J_A_C_S, where we try to make sense of the structure-reactivity patterns of isodiazenes that refuse to participate in nitrogen deletion. pubs.acs.org/doi/full/10.10…
Always a pleasure to continue to work with @UConnChem alumni! @TrevorAHamlin and I worked extensively on computational modeling during our grad school days and its great to be talking shop again with him and his team! Congrats to all involved and hopefully more to come soon!
This work has been published in @JOC_OL! 🖥️ 🧪 Congrats to the team! @YumanHordijk Baart Waaijer, @ckellzchem ! 🔥 doi.org/10.1021/acs.jo…
Today in @ScienceMagazine, we report a method to replace the C2 of pyridines with N, affording pyridazines. The change from electronically consonant (pyridine) to dissonant (pyridazine) opens retrosyntheses not typically available to the latter. science.org/doi/10.1126/sc…
Synthesis and Application of Geminal Halodiborylalkanes: Recent Advances and Future Perspectives (@ChemCatChem): …mistry-europe.onlinelibrary.wiley.com/doi/10.1002/cc….
Really enjoyed kicking off our 2025-2026 @JNJNews Spring House seminar series with @andrei_yudin from the University of Toronto. Andrei gave a great talk on the ‘dark space’ of macrocyclic peptide conformations & ‘frame-shifting’ our mentality to heterocyclic construction .…

Online today @NatureChemistry : nature.com/articles/s4155… Superstar Myojeong Kim has since started her lab at Yonsei - stay tuned for her next breakthrough!
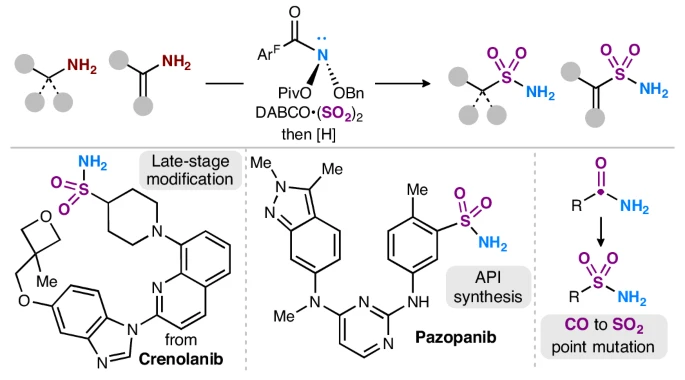
How do you turn a deletion into an insertion? In our latest, Myojeong shows that the anomeric amide can N-delete *and* re-aminate a sulfinate intermediate, enabling an overall SO2 insertion. Another great collab with @ckellzchem and J&J! doi.org/10.26434/chemr…
Super thrilled to finally see this collaborative work between @JNJNews 's DPR, CCAP, and ISD groups with @LevinChem and @group_levin live on @NatureChemistry ! Here's to more fruitful endeavors in the world of molecular editing and deaminative functionalization! Link:…
Words can't describe our feelings! In @ScienceMagazine, we present a flow method to prepare CF3-X anions on demand using organic precursors and CsF, avoiding #PFAS use in the process. 🔗science.org/doi/10.1126/sc… This allows for precise introduction of these groups at any stage!
A mechanistically interesting strain-release cascade reaction with BCBs ⚡️Synthesis of Oxa-spiro-bicyclo[2.1.1]hexane by Au/Sc Relay Catalysis | Organic Letters pubs.acs.org/doi/10.1021/ac…
Persistent Boryl Radicals as Highly Reducing Photoredox Catalysts for Debrominative Borylations | Journal of the American Chemical Society @BristolUni @TotalSyntheses pubs.acs.org/doi/10.1021/ja…
Our latest is a serendipitously discovered pair of reactions that can pull either the C2 or C3 carbon out of quinolines by choice of an amine scavenger. A deep mechanistic dive took us some surprising places, in @ChemieJisoo's latest. pubs.acs.org/doi/full/10.10… @alecchristian14
Brush up on your lab safety at an upcoming workshop, ACS CHAS Empowering Academic Researchers To Strengthen Safety Culture happening on Thursday, May 29, during the ACS Mid-Atlantic Regional Meeting (MARM) at Seton Hall University! eventbrite.com/e/acs-chas-emp…
Tonight is our final seminar of the 2024-2025 season. Closing things out we have THE @PatrickFier of Merck coming to talk about new chemical methods developed in industry!
Very fruitful collaboration with @Malins_Lab and @BaranLabReads on making BCPs without propellane! In @ChemRxiv: bit.ly/43ar8lC Congrats to Andrii (@Anslob11) and Flynn (@flynn_attard) for the heavy lifting!
New perspective on BCB-derived scaffolds 🏗️ Recent Advances in Catalytic Asymmetric Transformations of Bicyclobutane: A Versatile Building Block for Enantiopure Bioisosteric Molecules | ACS Catalysis pubs.acs.org/doi/10.1021/ac…
Check out @YumanHordijk’s fresh preprint on understanding radical addition reactions at C=X bonds! 🔥 Great collab with @ckellzchem ! 🤝 chemrxiv.org/engage/chemrxi…
Postdoctoral researcher Alexander Fanourakis and the Mark Levin lab have surpassed a major bottleneck in molecular building. Their new "placeholder" method dramatically streamlines the creation of key molecules like pyrazoles (used in drugs and agriculture) by ensuring a much…
So great to partner with Enamine again. I can personally vouch for this product - they sent us a sample to road-test and it was just as good as home-made.
🚀 t-Butyl N-{[3,5-bis(trifluoromethyl)benzenesulfonyl]oxy}carbamate — a next-generation reagent redefining what’s possible in heterocycle synthesis. This innovative molecule enables precise, regiocontrolled construction of N-alkylpyrazoles via a cutting-edge single-atom…
🚀 t-Butyl N-{[3,5-bis(trifluoromethyl)benzenesulfonyl]oxy}carbamate — a next-generation reagent redefining what’s possible in heterocycle synthesis. This innovative molecule enables precise, regiocontrolled construction of N-alkylpyrazoles via a cutting-edge single-atom…