NEJM Evidence
@NEJMEvidence
NEJM Evidence, a new journal from NEJM Group, presents innovative #OriginalResearch and fresh, bold ideas in #ClinicalTrial design and clinical decision-making.
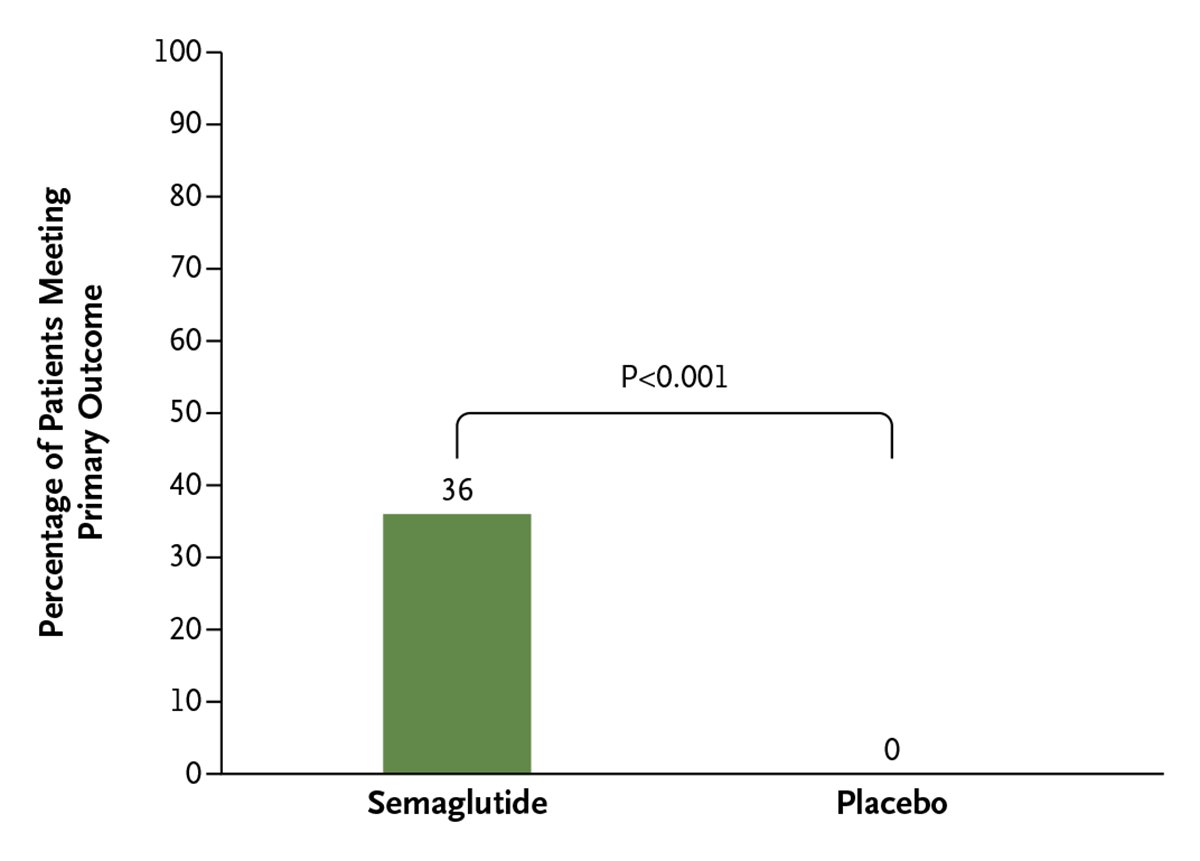
Volume 4 Issue 8 of 𝘕𝘌𝘑𝘔 𝘌𝘷𝘪𝘥𝘦𝘯𝘤𝘦 is now available! Here is a preview of the latest content: 𝗢𝗿𝗶𝗴𝗶𝗻𝗮𝗹 𝗔𝗿𝘁𝗶𝗰𝗹𝗲𝘀 Semaglutide in Adults with Type 1 Diabetes and Obesity eviden.cc/43YIrGt

Editorial: “Blocking Human-to-Mosquito Transmission of Plasmodium falciparum — A New Path to Malaria Elimination?” by Felix Bongomin, MSc, MMed, and Davidson H. Hamer, MD eviden.cc/4eizm0f #IDTwitter

Original Article: “A Vaccine to Block 𝘗𝘭𝘢𝘴𝘮𝘰𝘥𝘪𝘶𝘮 𝘧𝘢𝘭𝘤𝘪𝘱𝘢𝘳𝘶𝘮 Transmission” by S.A. Healy et al. eviden.cc/46a8CN7 #IDTwitter #GlobalHealth
“In May 2023, my first granddaughter, Arlynn, arrived. It was such a thrill, as I’m the mother of three sons. In October 2023, my second granddaughter, Hailey, arrived. I was elated. Whatever heart failure was, I had two phenomenal reasons to beat it.” eviden.cc/44etBfh

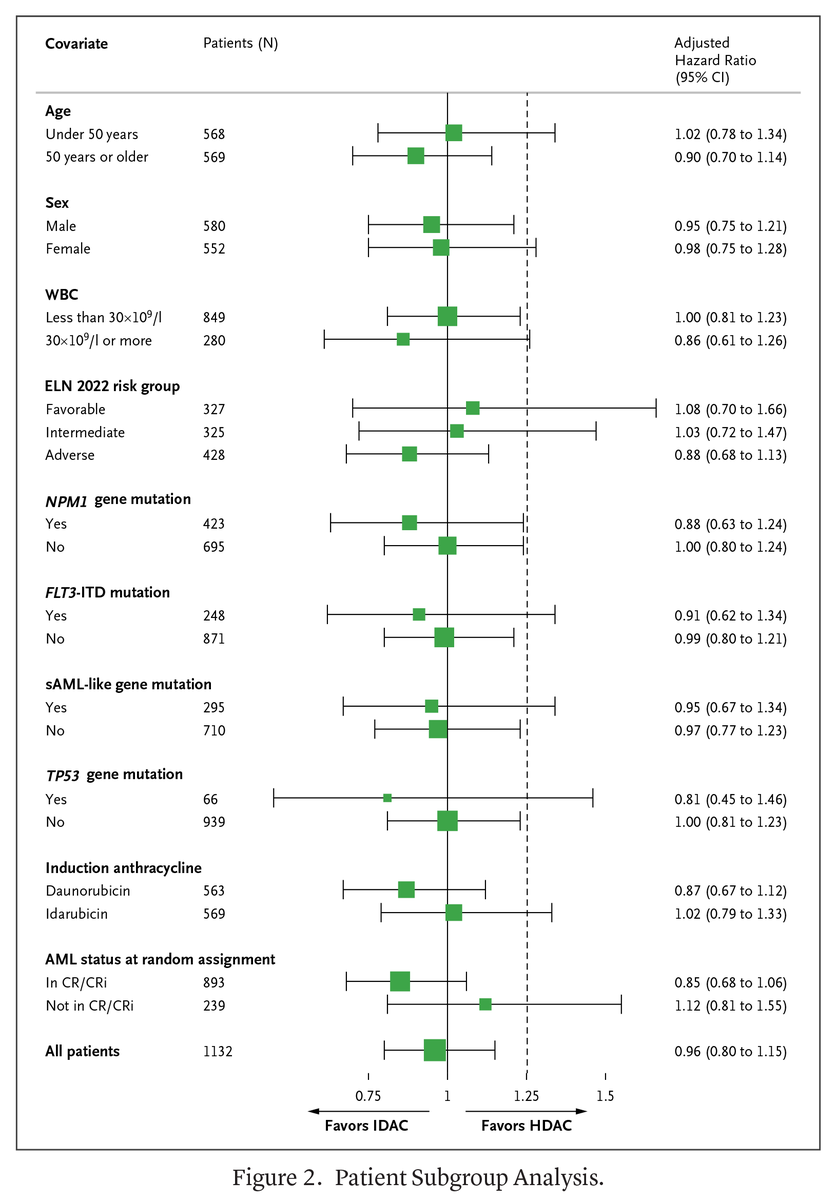
Original Article: “Intermediate-Dose Cytarabine as Postinduction AML Therapy” by M. Hunault et al. eviden.cc/4kU9Kcz #Hematology #ClinicalTrials

In adults with T1DM and obesity, addition of semaglutide, compared to use of automated insulin delivery alone, significantly improved achievement of a composite of time-in-range of >70%, with time below range of <4%, and a 5% body weight reduction. eviden.cc/43YIrGt
Original Article: “Sulopenem versus Amoxicillin/Clavulanate for the Treatment of Uncomplicated Urinary Tract Infection” by S. Puttagunta et al. eviden.cc/3T711r4 #IDTwitter #ClinicalTrials
“Recent advances in pacing technology have expanded the options beyond traditional systems, underscoring the need to tailor pacemaker selection to optimize patient outcomes.” 🏥 Read the full review article: eviden.cc/4ee15iB #Cardiology


A Different Angle on Malaria Control: Blocking Transmission from People to Mosquitoes A vaccine that targets transmission of Plasmodium falciparum from humans to mosquitoes shows promise in early testing. jwat.ch/3Iv7LwZ @NEJMEvidence #IDTwitter
“Advances in medical therapies have undoubtedly played a major role, but a changing patient spectrum and changes in lung cancer management and diagnostic practices have likely also contributed.” Read the full editorial: eviden.cc/43Y5Klf #Oncology #CancerResearch

“One major finding of this study is the improvement in survival achieved for patients with early-stage lung adenocarcinoma over 20 years, with a 3-year [overall survival] rate of 84% reached in 2020 ...” Read the full study results: eviden.cc/3I9GUpN
![NEJMEvidence's tweet image. “One major finding of this study is the improvement in survival achieved for patients with early-stage lung adenocarcinoma over 20 years, with a 3-year [overall survival] rate of 84% reached in 2020 ...” Read the full study results: eviden.cc/3I9GUpN](https://pbs.twimg.com/media/GwJLTvhbAAAjMBi.jpg)
Editorial: “Resiniferatoxin and the Future of Cancer Pain Management — A Step Forward?” by @krishnabshahmd and Bilal Dar, MD eviden.cc/3GWVcK2 @BCMNeurosurgery @BCMAnesthesia

In this Patient Platform, a new grandmother shares her experience receiving a diagnosis of heart failure – and all the prescriptions for new medications that came with it. Read "What Do You Mean, I Have Heart Failure?” by Nancy Figueroa: eviden.cc/44etBfh

Phil, maybe someday 𝘺𝘰𝘶'𝘭𝘭 be functionally independent. In a new video in our partnership with @DGlaucomflecken, the EXPECTS trial assessing alteplase for posterior circulation ischemic stroke at 4.5 to 24 hours is explained. Access the article for free:…
“...the integration of transmission-blocking vaccines into comprehensive, cross-sectoral strategies could mark the beginning of the end for one of humanity’s oldest and deadliest diseases.” Read the full editorial: eviden.cc/4eizm0f

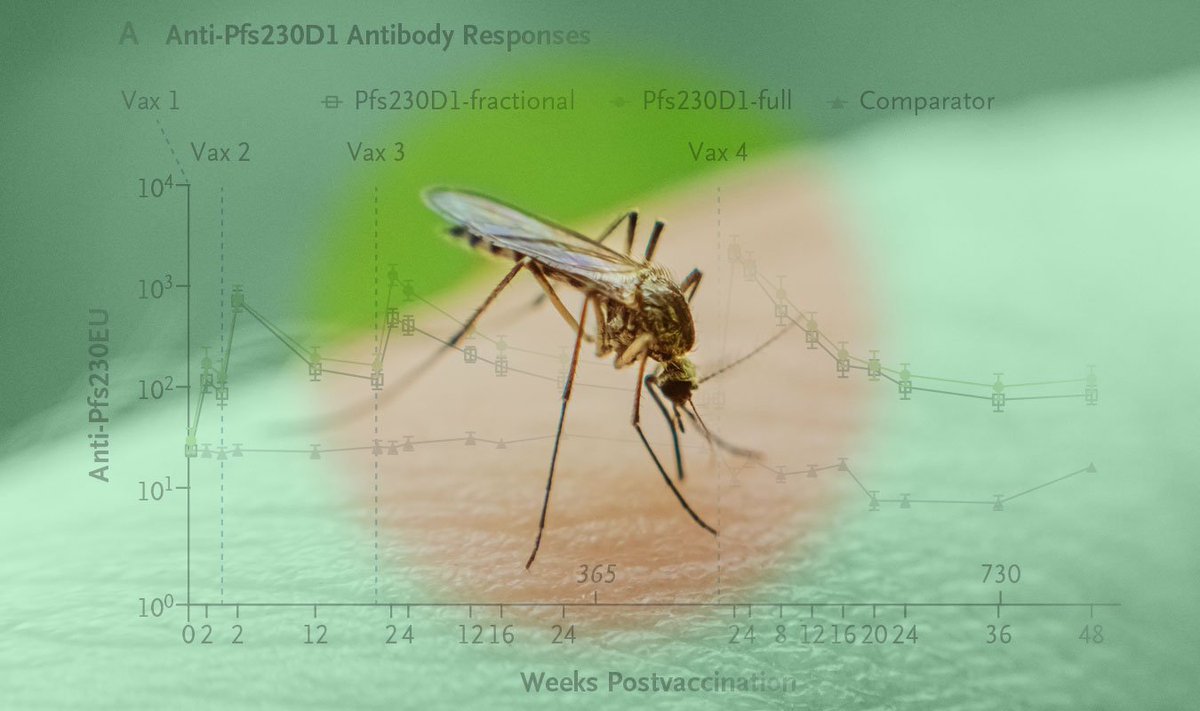
“In this trial, Pfs230D1-EPA/AS01 regimens did not result in [serious adverse events] and generated antibody responses and functional activity that persisted for up to 1 year postvaccination.” Read the full trial results: eviden.cc/46a8CN7
![NEJMEvidence's tweet image. “In this trial, Pfs230D1-EPA/AS01 regimens did not result in [serious adverse events] and generated antibody responses and functional activity that persisted for up to 1 year postvaccination.” Read the full trial results: eviden.cc/46a8CN7](https://pbs.twimg.com/media/Gv_EuFXaIAIUadX.png)