Matt Galsky
@MattGalsky
I am a medical oncologist, Director of Genitourinary Medical Oncology @TischCancer and Professor of Medicine @IcahnMountSinai. Views are my own.
Tweetorial here on new story related to resistance to PD-1/PD-L1 blockade in bladder cancer. @BhardwajLab @CancerResearch @parkerici @TischCancer @BladderCancerUS @DrJohnSfakianos @AmirHorowitz @AACR @UroDocAsh @tompowles1 @montypal @ERPlimackMD clincancerres.aacrjournals.org/content/early/…
Should #radicalcystectomy ever be considered for consolidate therapy post EV/P?dailynews.ascopubs.org/do/personalizi… @PGrivasMDPhD @shilpaonc @tompowles1 @MattGalsky @OncBrothers @montypal @DrYukselUrun @nirajaiims @UroDocAsh @IBCG_BladderCA @SWOG @UroOnc @BladderCancerUS
ctDNA can help guide adjuvant decision making in MIBC In pts with undetectable ctDNA after NAC + cystectomy, no definitive benefit for adjuvant ICB authors.elsevier.com/c/1lMET14kpm0h… @MattGalsky @TischCancer @IcahnMountSinai @Uromigos @BladderCancerUS @urotoday @WorldBladderCan @OncLive
Out in NEJM!! 🚨 Breakthrough in HLRCC-associated RCC! A Phase 2 trial shows that 💉bevacizumab + erlotinib offers high efficacy in advanced papillary renal cell carcinoma: 🔬 HLRCC-associated pRCC: ✅ 72% response rate ⏱️ Median PFS: 21.1 mo 💀 Median OS: 44.6+ mo 🔬 Sporadic…
🔬 CONTACT-02 Phase 3 Trial in mCRPC 🔬 📍 Patients with metastatic castration-resistant prostate cancer + extrapelvic soft-tissue mets post-ARPI 💊 Cabozantinib + Atezolizumab vs ARPI switch ✅ PFS: 6.3 vs 4.2 mo (HR 0.65, P=0.0007) ❌ OS: No significant difference (14.8 vs…
An excellent review on optimising treatment of stage 2 seminoma. Very well written highlighting the double edge sword - efficacy and toxicity. doi.org/10.1200/OP-25-… @DrChoueiri @urotoday @DrRosenbergMSK @BradMcG04 @DrKarineTawagi @DrRanaMcKay @MattGalsky @BhardwajLab…
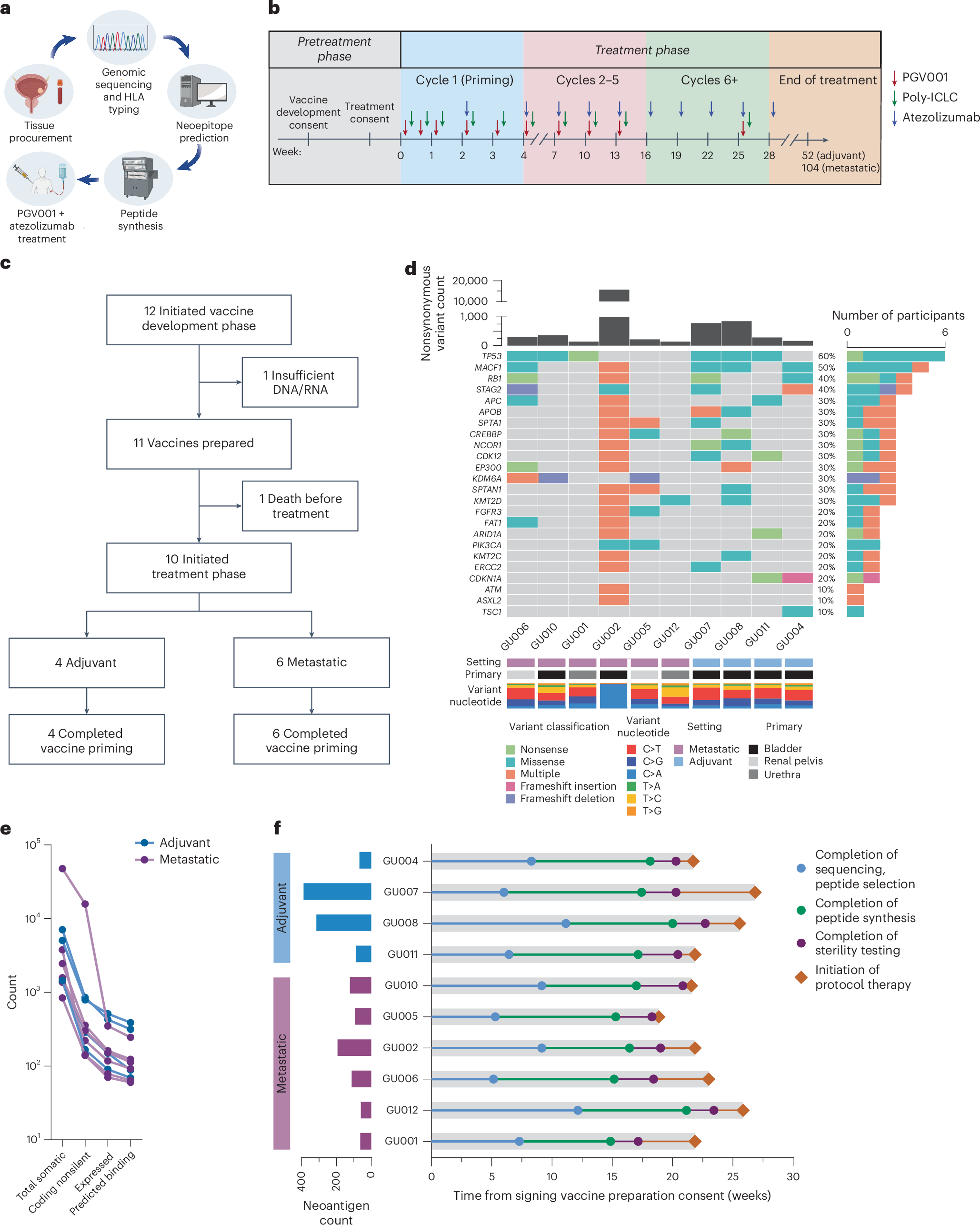
Saxena, Anker, Kodysh et al. demonstrated that the personalized long-peptide neoantigen vaccine PGV001, in combination with atezolizumab, was feasible, safe, and elicited durable neoantigen-specific T cell [...] bit.ly/3T6h5cq @IcahnMountSinai @JuliaKodysh @MattGalsky
Very proud of our own @SaadAtiq24 @theNCI senior fellow presenting data on the Prevalence of Histology-Agnostic Biomarkers in Pure Squamous Cell Carcinomas of the #Bladder showing 1/3 of patients with TMB-High @ASCO #ASCO25 @carisls
Trying to reconcile the ctDNA detection rates post-RC from NIAGARA versus IMvigor 010 and other datasets...remember IMvigor 010 only included patients with high risk path features (whereas post RC data in NIAGARA includes all ≥ypT0N0). #MedIQASCO25 #ASCO25 #bladdercancer.
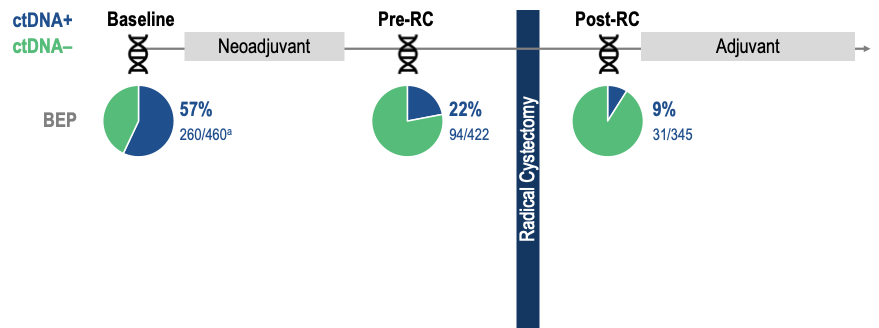
Congrats @FaltasLab for the CLONEVO study presentation showing us the ctDNA can help distinguish "contribution of components" in WOO studies in MIBC. Another piece of elegant work from his group. #MedIQASCO25 #ASCO25 #bladdercancer
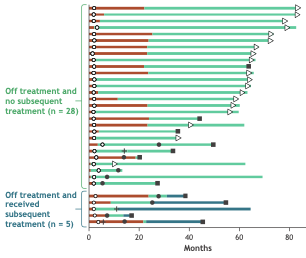
Our contemporary "negative" RCTs in metastatic UC are just not the same as our "negative" RCTs of the past. Granted a small subset -- but this is durable disease free survival OFF treatment after Ipi + Nivo #CM901. #MedIQASCO25 #ASCO25 #bladdercancer.
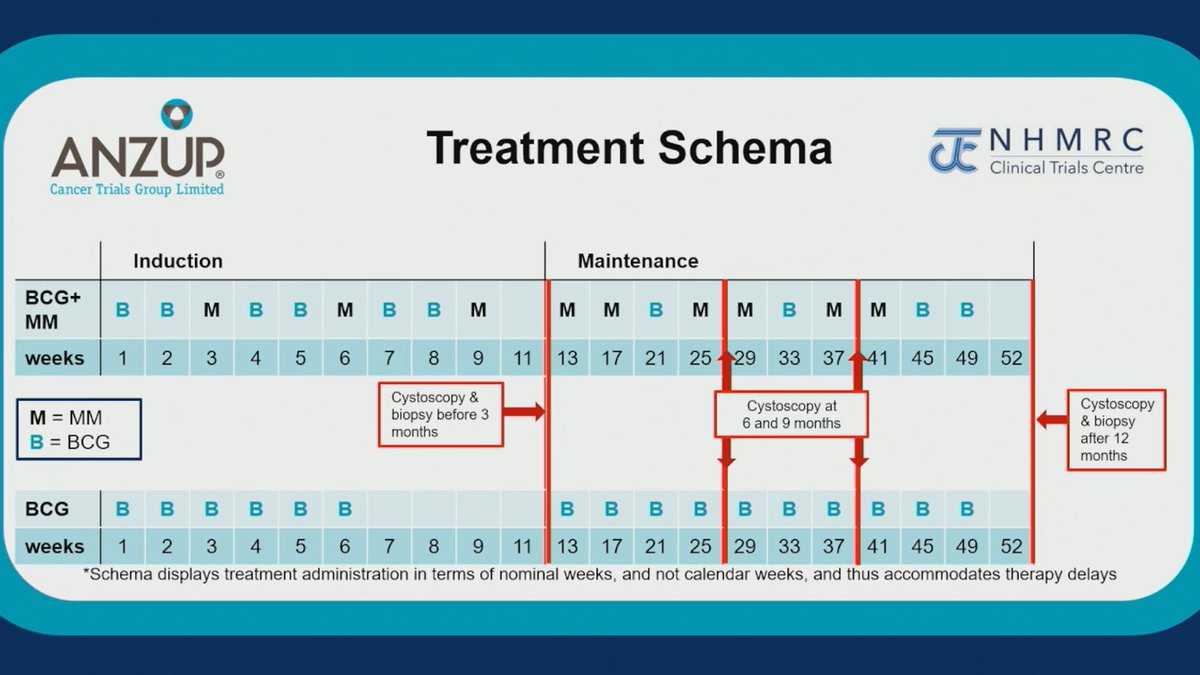
Important academic study in context of BCG shortage. No definitive benefit for adding mitomycin but ?decreased progression and benefit in patients at highest risk (2 endpoints/subsets). #MedIQASCO25 #ASCO25 #bladdercancer Congrats @ANZUPtrials
@MattGalsky discusses the ctDNA data from NIAGARA presented at #ASCO25. ctDNA positivity is distinct from path CR and ctDNA positive patients do poorly creators.spotify.com/pod/show/the-u…
@MattGalsky @IcahnMountSinai leads @ALLIANCE_org A032103 (MODERN) to test the role of DNA released from tumor cells into the blood in guiding the use of #immunotherapy after bladder removal for #bladdercancer treatment: bit.ly/Alliance-A0321… @bladdercancerus
@ASCO is where bold ideas meet breakthroughs. Ahead of #ASCO25, we’re highlighting the MODERN trial, investigating using ctDNA to guide post-cystectomy immunotherapy in bladder cancer. Watch the IRB-approved video: primrmed.com/cancer-clinica… @ALLIANCE_org @NateraGenetics @theNCI
Thrilled to share our new analysis from CheckMate 901! 🟡In N+ only metastatic UC, Nivolumab + chemo doubled complete response rate vs chemo alone (63% vs 34%) and prolonged PFS (HR 0.38) and OS (HR 0.58). ➡️A new horizon for a distinct subgroup. @EUplatinum @OncoAlert…
Just in case anyone missed this? prnewswire.com/news-releases/… @urotoday @Uromigos @BladderCancerUS

JUST IN/Big news for #bladdercancer: PDL1 inhibitor Durvalumab regimen demonstrated improvement in DFS for high-risk non-muscle-invasive bladder cancer in POTOMAC Phase III trial. News from @AstraZeneca this morning! @BladderCancerUS @DanaFarber_GU astrazeneca.com/media-centre/p…
Atezolizumab plus personalized neoantigen vaccination in urothelial cancer: a phase 1 trial OUT ON nature Cancer nature.com/articles/s4301… Study evaluated the feasibility and safety of combining atezolizumab, an anti–PD-L1 immune checkpoint inhibitor, with PGV001, a personalized…
We tested the feasibility and immunogenicity of a personalized neoantigen vaccine + PD-L1 blockade in #bladdercancer including the adjuvant setting. nature.com/articles/s4301… @JonAnker1 @BhardwajLab @urotoday @Uromigos @TischCancer @FaltasLab @NatureCancer @BladderCancerUS
Why is there a difference in the pCR from NIAGARA at #ASCOGU25 @MattGalsky & #AUA25 @JoshMeeks (37% vs 42% for chemo/durva)? The 37% includes all ITT patients while the 42% only has surgery pts. Variability in pCR reporting is widespread making cross trial comparison hazardous.⚠️
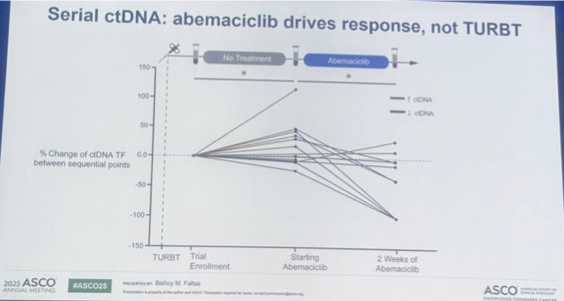
Stellar neoadjuvant/adjuvant clinical trials session today #AACR25 @AACR chaired by @aparna1024! @FaltasLab presented #CLONEVO trial, neoadjuvant abemaciclib for cis-ineligible #bladdercancer - we participated @utswcancer led by @SuzanneColeMD!