
Hillary Andrews, PhD
@HillStirSci
passionate about improving cancer research and policy with and for patients || director, regulatory & research partnerships @cancerresrch || tweets = my own
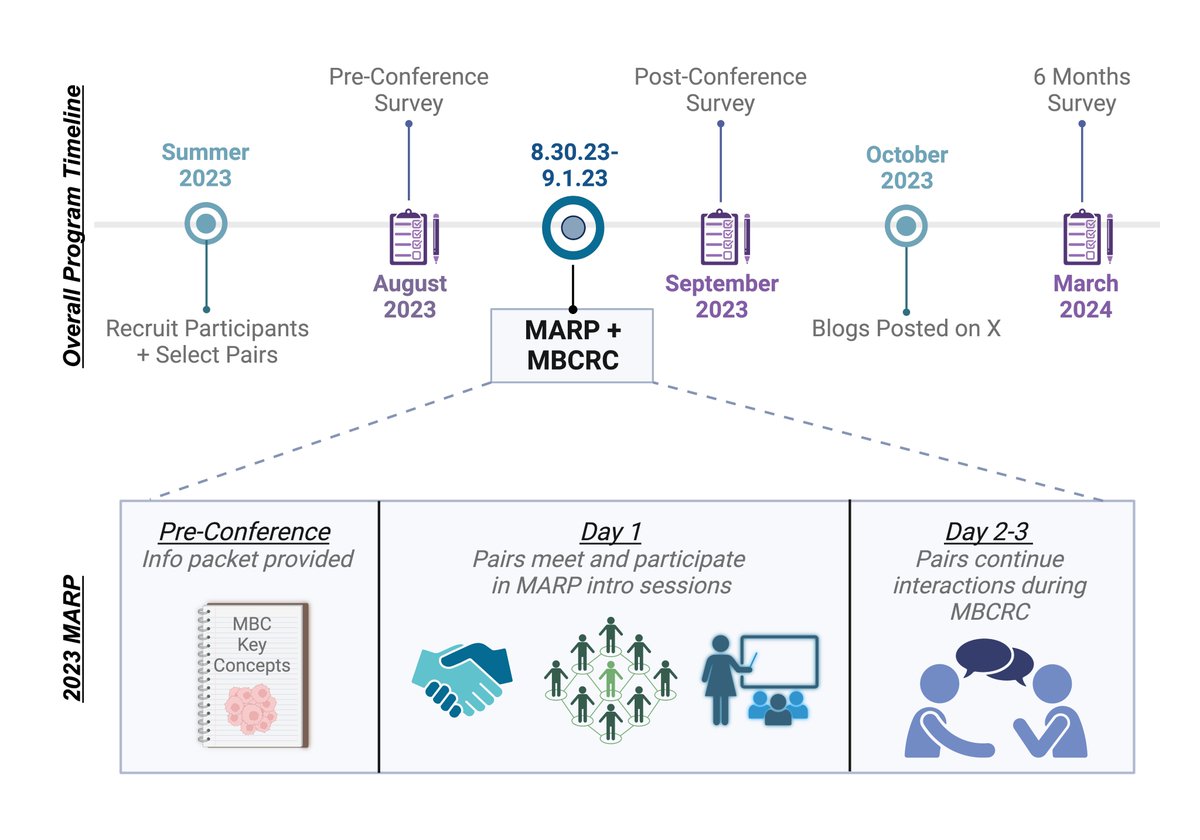
Our manuscript highlighting the design, goals, and experiences of the MBCRC Advocate Researcher Program (MARP), which focused on building meaningful, bidirectional partnerships between advocates and researchers was published in @Nature_NPJ breast. nature.com/articles/s4152…
PCDI @the_rightdose is again collecting data on the experience of people with MBC regarding treatment, tolerability & QoL. Our latest survey focuses on those who have taken an Antibody-Drug-Conjugate (approved or in a clinical trial). 📢 Take the survey & make your voice heard!
Attending #SABCS24? Join the @GRASPtweets reception, one of the few opportunities for scientists, clinicians & patient advocates to connect. It's where so many collaborations are born to bring the patient experience to research! REGISTER at graspcancer.org/events/sabcs24…
Advocates: it’s your turn to register! And don’t delay - many sessions have sold out already! (Sign up for waitlist if you like one that’s full - people always cancel and you’ll automatically get in!)
📣 Registration is open for all Virtual GRASP Sessions for SABCS2024. The sessions are virtual, and spaces will fill up quickly, so register today! graspcancer.org/events/sabcs24/ #grasp #graspsessions #registernow #sabcs2024 #virtualsessions
Our manuscript is out! Thank you to all the researchers and advocates who contributed to this work. einpresswire.com/article/824868… Special shout out to @HillStirSci for her leadership. @Nature_NPJ #cancerresearch
Absolutely excellent, such a fantastic programme. 👇🏻is @DieNicole2 & my learning etc. josh-newby-2p7g.squarespace.com/marp/metarp-20… @DieNicole2
Rare #cancer biomarkers make CDx validation challenging due to limited samples. Our new @DrugInfoAssn article proposes FDA guidance recommendations to clarify appropriate use of alternative samples—ensuring patient access to safe and effective treatments: bit.ly/3F5vP8i
So excited to see this work published!!
New @CancerReserch findings in @CCR_AACR aggregating and assessing 8 clinical trials of patients with #aNSCLC treated with TKI indicate circulating tumor DNA (#ctDNA) clearance on treatment is associated with improved overall survival. Learn more: bit.ly/4j7pLe9
Looking to include more advocate partners in your work, or train as an advocate yourself? @TanjaSpanic @EuropaDonnaSLO includes this list of for places to start. #SGBCC2025
📍Final day (2/28/25)📍to take the PCDI (Patient-Centered Dosing Initiative) ADC survey! 🙌 @ 196 responses‼️ (goal is 200), thanks to all who’ve taken the survey!🙌 👉🏻🇺🇸 US pts w/ MBC currently/formerly on an ADC, email [email protected] for survey link! #bcsm #QoL
2 days remaining until the Patient-Centered Dosing Initiative (PCDI) ADC survey closes (3/1/25). We’re SO close to our goal of 200 responses - if you’ve not taken the survey, & are eligible, please take 20 minutes to help us gather data on the pt experience! ✔️Must reside in…
Advocates! @CancerResrch's advocate focused virtual event that provides a deeper dive into our discussions from last week's diagnostics meeting is February 19! Register today and share your questions - we'd love to answer them during the event! friendsofcancerresearch.org/event/advancin…
Interested in learning more about AI, diagnostics, and regulatory science as discussed in Session 3? Explore our newly launched Policy Priorities Page: friendsofcancerresearch.org/policy-priorit…. #FriendDx
It was so incredible to work with such a great group of experts to align on the output of this white paper. Regulatory flexibilities for companion diagnostics with limited clinical samples are important to support patients receiving access to novel targeted therapy in oncology.
Read more about the Innovative Validation and Regulatory Processes for Companion Diagnostic Tests for Rare Biomarkers or Indications white paper discussed in Session 2: bit.ly/4hbeuIU #FriendDx
At the end of the day, patients deserve high quality tests especially when the test determines the drug that will prolong their life. - Elizabeth Mansfield from @FoundationATCG #FriendDx
Learn more about the Session 1 findings in the digital pathology discussion document (bit.ly/3Egbqwm) and explore our Digital PATH Project research (bit.ly/3Kf4xes). #FriendsDx
The Advancing the Future of Diagnostics and Regulatory Innovations meeting is live! Stream on YouTube now or later on-demand: youtube.com/live/wFHolEBYx…. #FriendDx
In 2024, Friends’ made pivotal strides in advancing #oncology drug development, regulatory policy and promoting innovative trial designs, contributing to advancements in therapy development, evaluation, and delivery. Read Friends' 2024 Scientific Report: Bit.ly/4gCLh9u
At the Advancing the Future of Diagnostics meeting on 2/4, a panel will discuss Friends’ whitepaper on innovative validation processes for companion #diagnostictests in rare disease settings. Read the full paper before the meeting: bit.ly/4hbeuIU.
Hey #bcsm, join me in congratulating @stales on her promotion at @Medidata to CHIEF PATIENT OFFICER. We've known she was destined for great things when she created one of the strongest communities here. Can't wait to see where she will go now! 🚀🎉
Starting now! @CancerResrch's webinar providing an overview of the 3 topics from the recent #Friends’ Annual Meeting 2024 and offering #advocates the opportunity to engage directly with experts. #FriendsAM24. bit.ly/3DaVx9P