
Friends of Cancer Research
@CancerResrch
Friends of Cancer Research powers advances in science and policy that speed life-saving treatments to patients.
Building trust in #AI for #cancercare requires benchmarks that capture real-world representativeness and clinical realities. In our response to @theNCI, we outline key priorities to help AI tools meet regulatory standards and deliver reliable results: bit.ly/40Aju3q.

FDA-approved cancer drugs frequently require additional postmarketing studies to address outstanding safety and efficacy questions. Our PMR/Cs dashboard analyzes novel #oncology therapies approved from 2012-2024, revealing postmarketing landscape trends: bit.ly/4aIVfCx.
Lost in the language of drug development? Our ProgressforPatients.org course explains key regulatory terms like "Breakthrough Therapy designation" in plain language. Empower your advocacy with knowledge. #patientadvocacy

.@US_FDA’s ODAC voted the benefit-risk profile of 2 #multiplemyeloma combination therapies is not favorable due to concerns around high-rates of toxicity at the proposed dosages, inadequate dose optimization, and poor representation of U.S. patients. friendsofcancerresearch.org/blog/stakehold…

Many promising #celltherapies for rare diseases stall due to regulatory, manufacturing and access barriers. Our white paper proposes fit-for-purpose CMC frameworks, alternative manufacturing models and cost recovery approaches to expand access. bit.ly/3YYACir

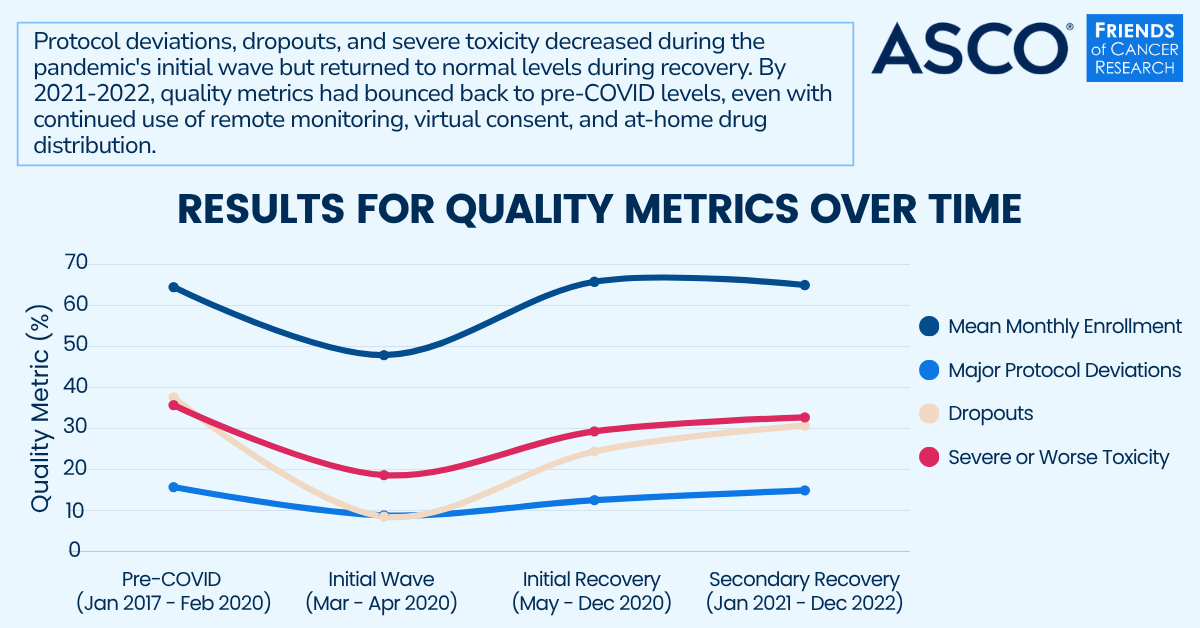
A @CancerResrch and @ASCO task force found that #clinicaltrial flexibilities can be implemented without compromising data quality, suggesting decentralized elements maintain standards while improving access and increasing enrollment: bit.ly/4kIRwKb.


Stay informed on the latest developments in #cancerresearch, policy, and regulatory science. Sign up for our newsletter for research updates, event announcements, and opportunities to engage in patient advocacy efforts that drive change: bit.ly/44mUJtM.

We are encouraged by the news that the @US_FDA and @NIH released a glossary of RWD/#RWE terms to help standardize definitions to strengthen communication across studies using innovative trial designs and data sources. Read the full story at @RAPSorg: raps.org/News-and-Artic….
Pragmatic #clinicaltrial elements (i.e. broadening eligibility criteria, utilizing real-world evidence, and embedding trials in routine care) can reduce patient burden, enhance generalizability, and maintain scientific rigor. Read more: bit.ly/4k3KXBm.

Using publicly available data from review documents on Drugs@FDA, we created a dashboard to assess how four of @US_FDA’s expedited programs impact the time it takes for new cancer therapies to be approved. Explore the dashboard: bit.ly/4kZJ6yX. #acceleratedapproval
.@balasubramaniac, @Path_AI, on how advancing AI/ML-enabled #digitalpathology tools requires collaboration to enhance accuracy & reproducibility. Our discussion guide explores how use of reference datasets can be an efficient approach toward validation: bit.ly/3Egbqwm.
#PatientAdvocates, your Q2 Advocate Newsletter is here! Get highlights from our recent conference, trends in Accelerated Approval and breaking news in cancer research. Read the latest issue and subscribe to stay up to date on cancer advocacy and research: friendsofcancerresearch.org/blog/quarterly….

Attending @IAmBiotech International Convention? Jeff Allen will discuss recent trends in Accelerated Approvals and how the mechanism continues to support timely patient access to treatments for serious illness. #BIO2025

New framework developed in partnership with @parkerici highlights the need for flexible approaches to efficiently characterize & advance cell therapies. This will enable rapid prioritization of enhanced T-cell constructs. Read @jitcancer manuscript here: bit.ly/440TgrI

Attending #DIA2025? Hillary Andrews chairs a panel on validation approaches and regulatory flexibilities for rare biomarker diagnostics, which will discuss strategies for leveraging alternative data sources. Learn more about this work: bit.ly/4dT5bwc.

Mark Stewart, VP of Science Policy, will join a panel at @DrugInfoAssn's Annual Meeting to discuss AI/ML-enabled diagnostic tools, exploring key challenges, opportunities, and regulatory considerations. Learn more about this work: friendsofcancerresearch.org/digital-pathol…. #DIA2025

A new analysis published in @ASCO #JCOOA finds that flexibilities implemented in cancer clinical trials during the COVID-19 pandemic did not compromise data quality, supporting the broader adoption of decentralized trial elements. Read the full analysis: bit.ly/44k3QeA

Jeff Allen will join two panels at @DrugInfoAssn's Annual Meeting to discuss novel approaches for testing and manufacturing cell and gene therapies and the accelerated approval program's role in delivering treatments for serious illnesses. See you at #DIA2025!

In 2020, we released a blueprint for strengthening the #AcceleratedApproval pathway while preserving scientific rigor. Recognizing its critical importance, Congress codified these priorities in the 2023 Consolidated Appropriations Act. Learn more: bit.ly/4dpz7jn.

Yesterday, Jeff Allen, President and CEO, participated in a Cell and Gene Therapy Roundtable hosted by @US_FDA Leadership to discuss enhanced regulatory support to advance novel treatments. Watch the full roundtable at youtube.com/live/0qDhRtnHX….
