
Amylou Dueck, PhD
@BiostatGirl
Statistical superhero. Professional data whisperer. Hall of fame golfer. And lover of bar charts (#sorrynotsorry). Tweets are my own.
“Enhancing clinicians’ and patients’ ability to read and interpret quality-of-life analyses will support a more complete and critical assessment of the true value of cancer treatments.” Read the Review Article by @MassimoDiMaio75: eviden.cc/431mUhd #ClinicalTrials
Listening for the journey home - new @TheLancetHaem podcast featuring a discussion of our commission on adverse events in haematology open.spotify.com/episode/5kT1Ya… #EHA2025
Folks at #EHA2025: check out our poster (PS1898) on PROs in EPCORE NHL-1. Patients with R/R FL who had 3+ symptoms at baseline had clinically-meaningful improvements in lymphoma-related symptoms that continued to improve after epco initiation. library.ehaweb.org/eha/2025/eha20…
🎧Tune in now to hear the authors’ insights directly open.spotify.com/episode/5kT1Ya…
For those missing the #EHA2025 fun, a #podcast discussing the @TheLancetHaem Commission topic of better adverse event assessment was just posted! buzzsprout.com/1564352/episod… @BroeckelmannPJ @majorajay, @GitaThanaMD (also features Vishal Bhatnagar & Lan-Lan Smith not on X)
For those missing the #EHA2025 fun, a #podcast discussing the @TheLancetHaem Commission topic of better adverse event assessment was just posted! buzzsprout.com/1564352/episod… @BroeckelmannPJ @majorajay, @GitaThanaMD (also features Vishal Bhatnagar & Lan-Lan Smith not on X)
Great to join @BroeckelmannPJ presenting data from our recent @TheLancetHaem commission on adverse events, starting with the paper by @majorajay @BiostatGirl on advancing AE measurement #EHA2025 @GitaThanaMD
Nice to see this EORTC QLQ-F17 publication! Sometimes you might want to measure the functioning domains by the EORTC library & side effects by PRO-CTCAE in your #ClinicalTrial. Using the whole QLQ-C30 creates too much overlap, so using the QLQ-F17 is one possible strategy!
🚀 New publication by Florian Zeman et al. shows that future clinical studies can use the @EORTC QLQ-F17 questionnaire as a generic tool without losing comparability to studies using the QLQ-C30. 🔗 doi.org/10.1016/j.ecli… #CancerResearch #QualityofLife #GivingVoice2Patients
Being a biostatistician on cancer clinical trials is the best job! I get to collaborate every day with so many amazing and inspired people including other biostatisticians, oncologists/ hematologists/ clinical trialists, patient research partners, and other experts! #ASCO25


PROs in the CABINET trial presented by me - cabozantinib for previously-treated advanced neuroendocrine tumors. Side effects & function declined, but HRQOL & overall status improved. Excited for more analysis! #ASCO25 @ALLIANCE_org
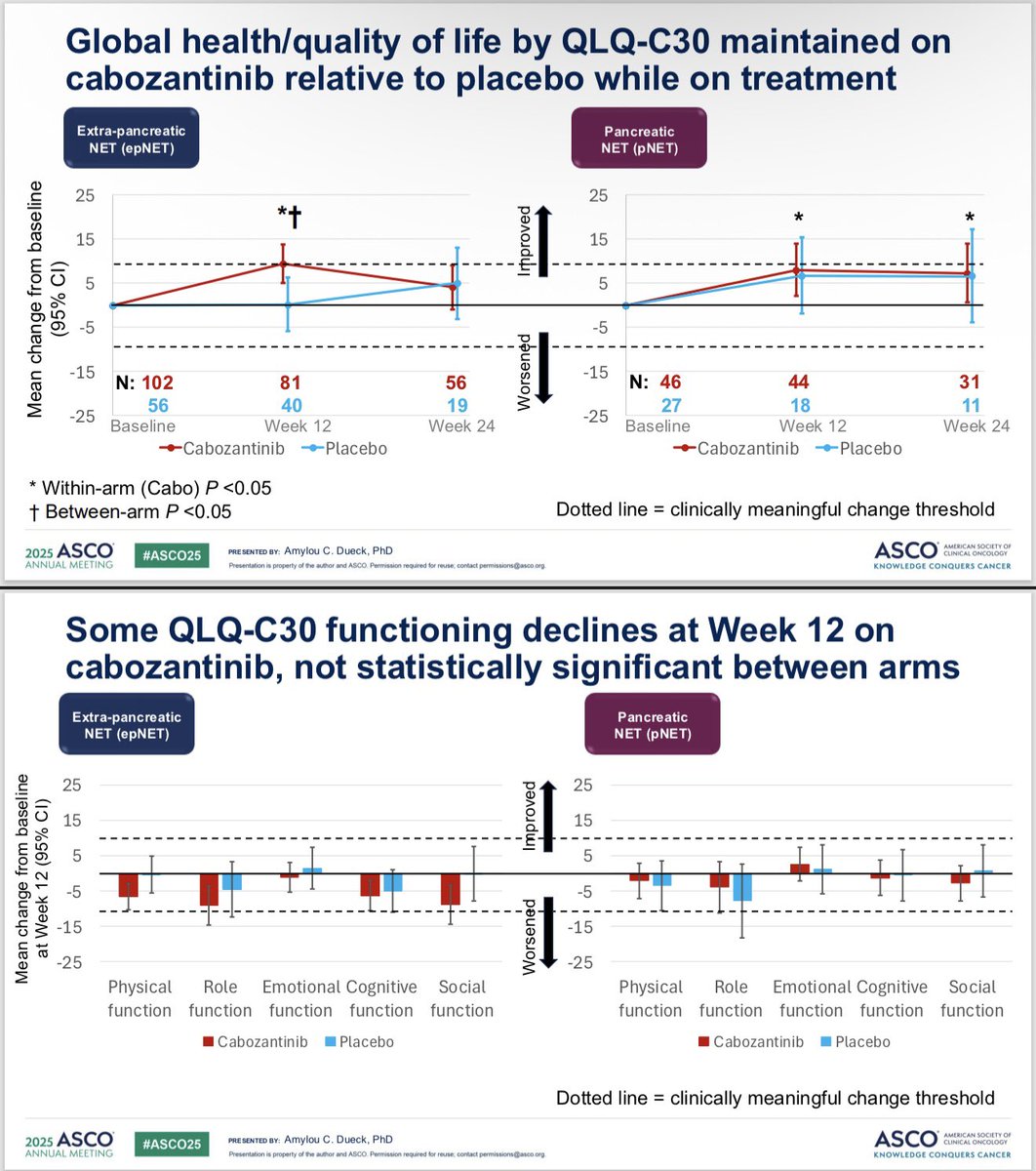
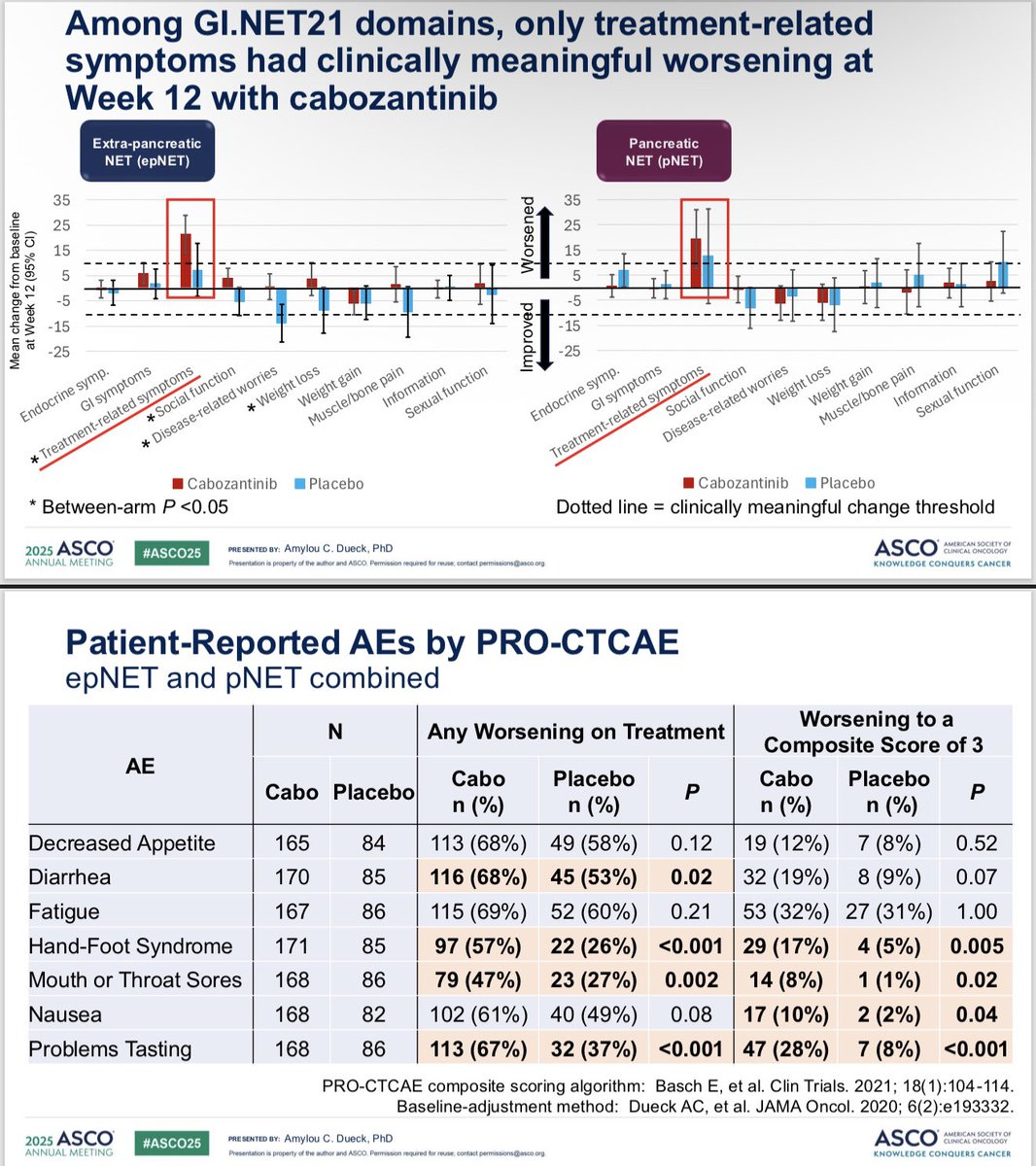
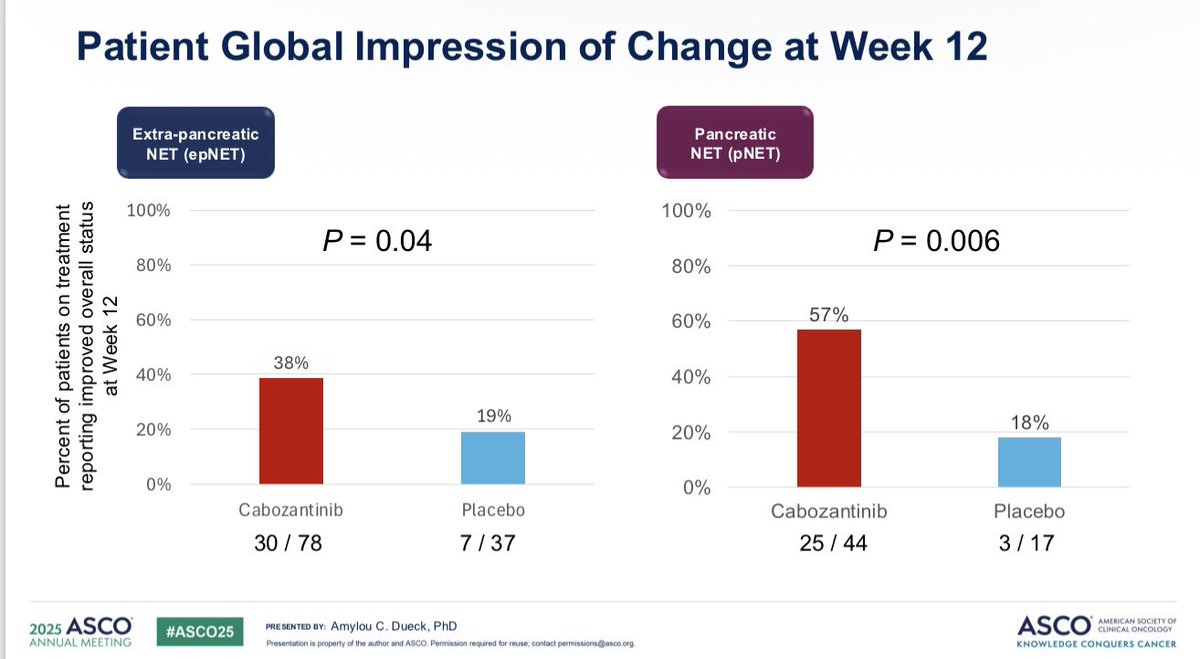

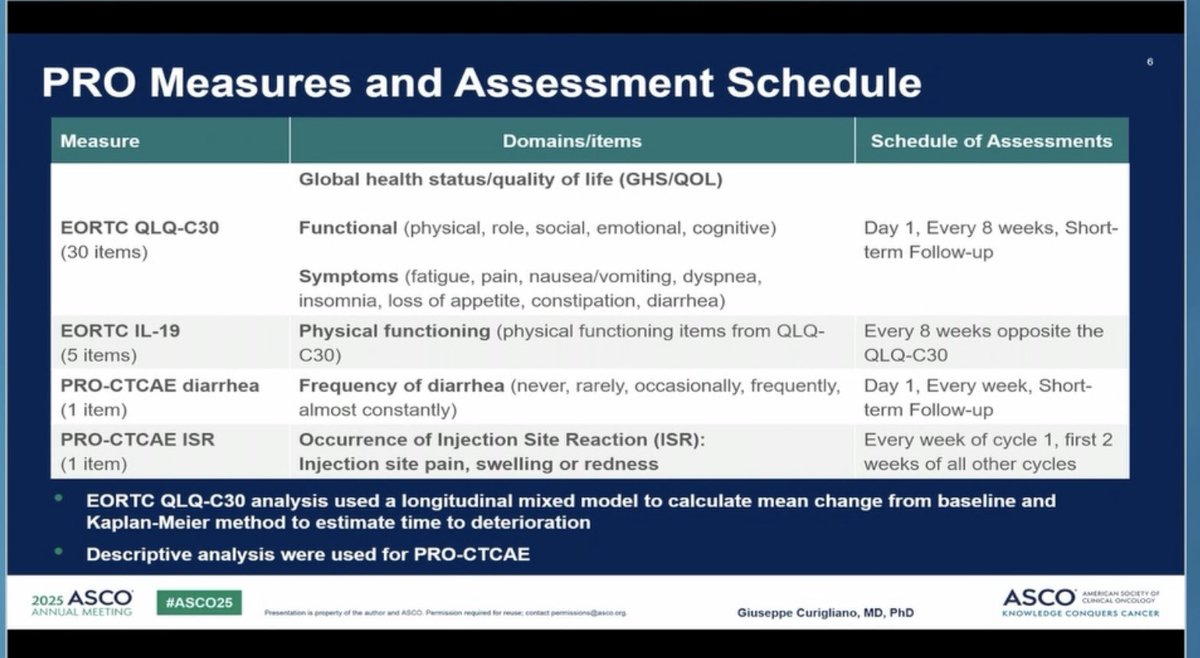
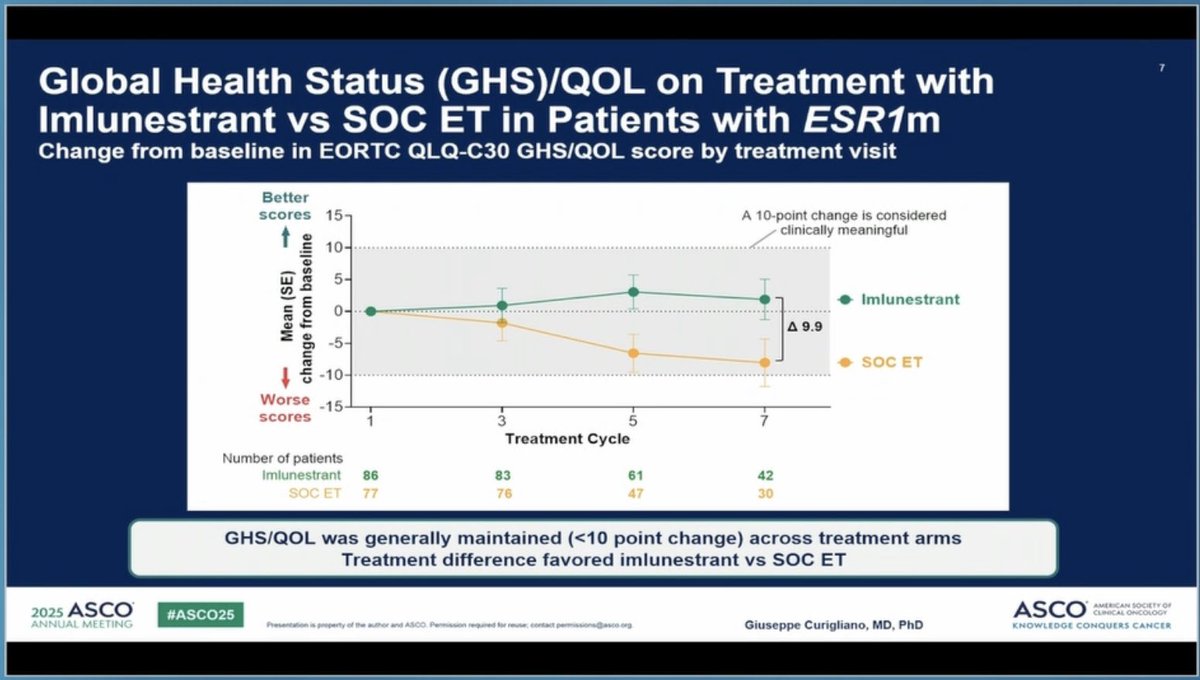
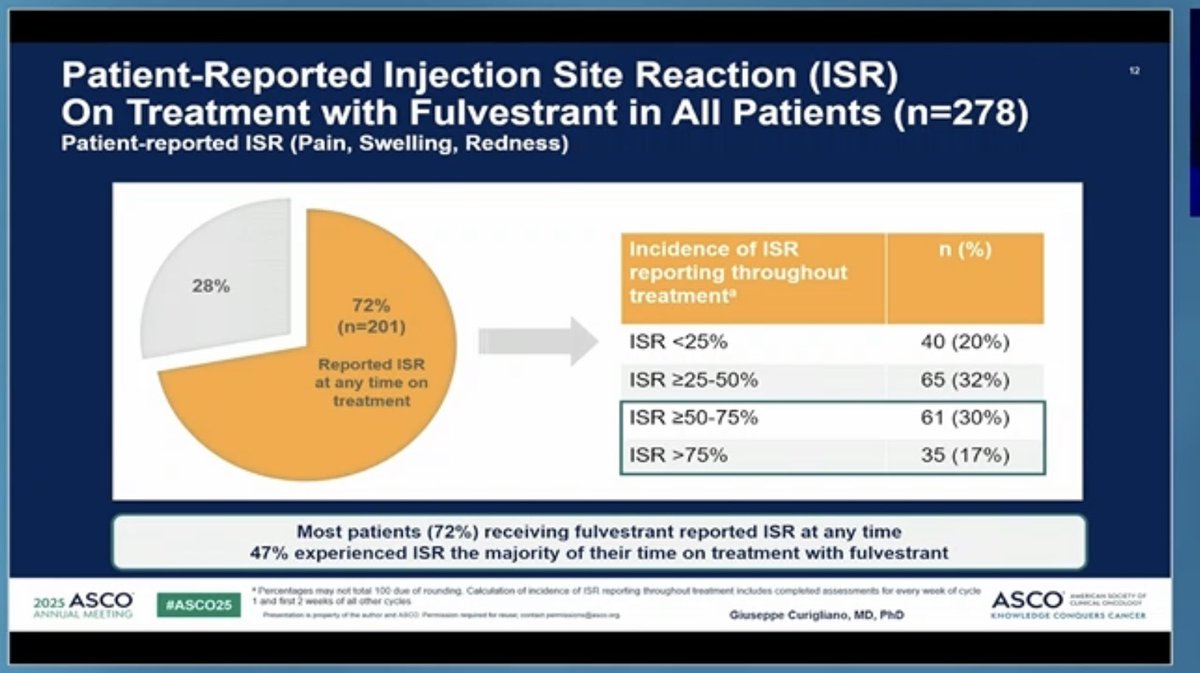
Kudos to #ASCO25 for including PROs in the big oral sessions! Nice presentation of EORTC and PRO-CTCAE data in EMBER-3 trial (imlunestrant vs SOC for ER+ advanced breast cancer) by Giuseppe Curigliano. Injection site reaction item of PRO-CTCAE featured prominently here!

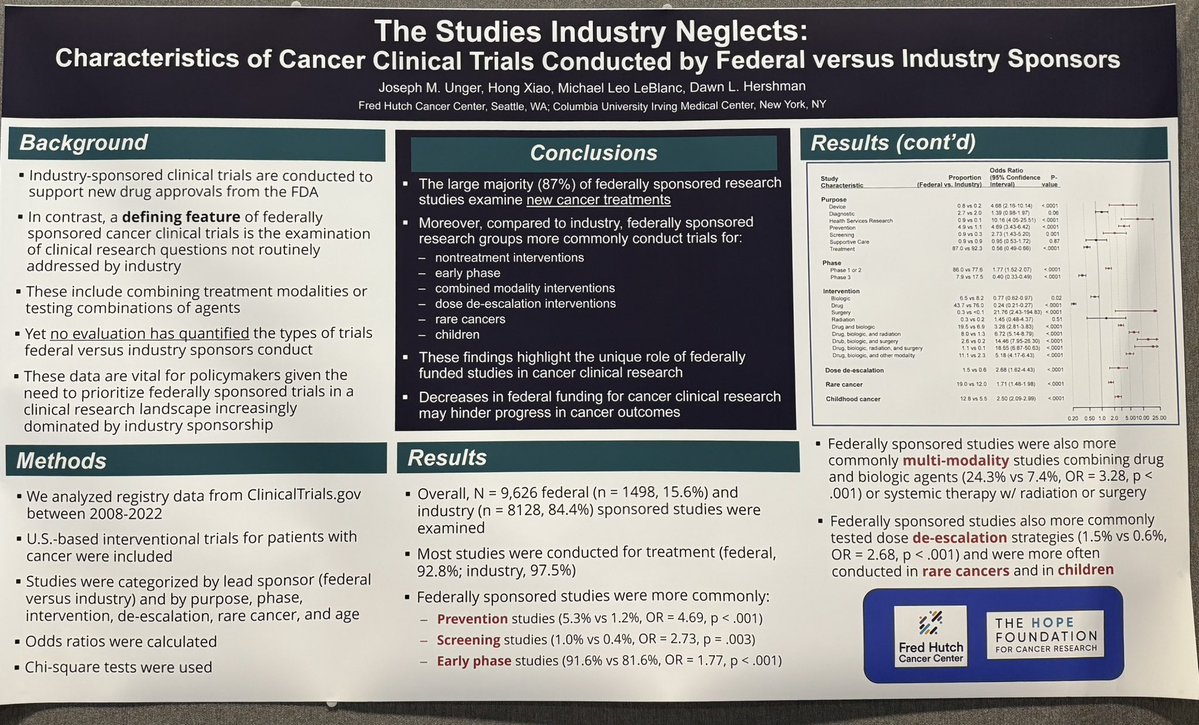
Cancer trials with a federal lead sponsor compared to industry sponsor are more likely to investigate de-escalation interventions, rare cancers, and children, among other things using data from clinicaltrials.gov from 2008-2022. #ASCO25 poster by Joe Unger at @fredhutch.
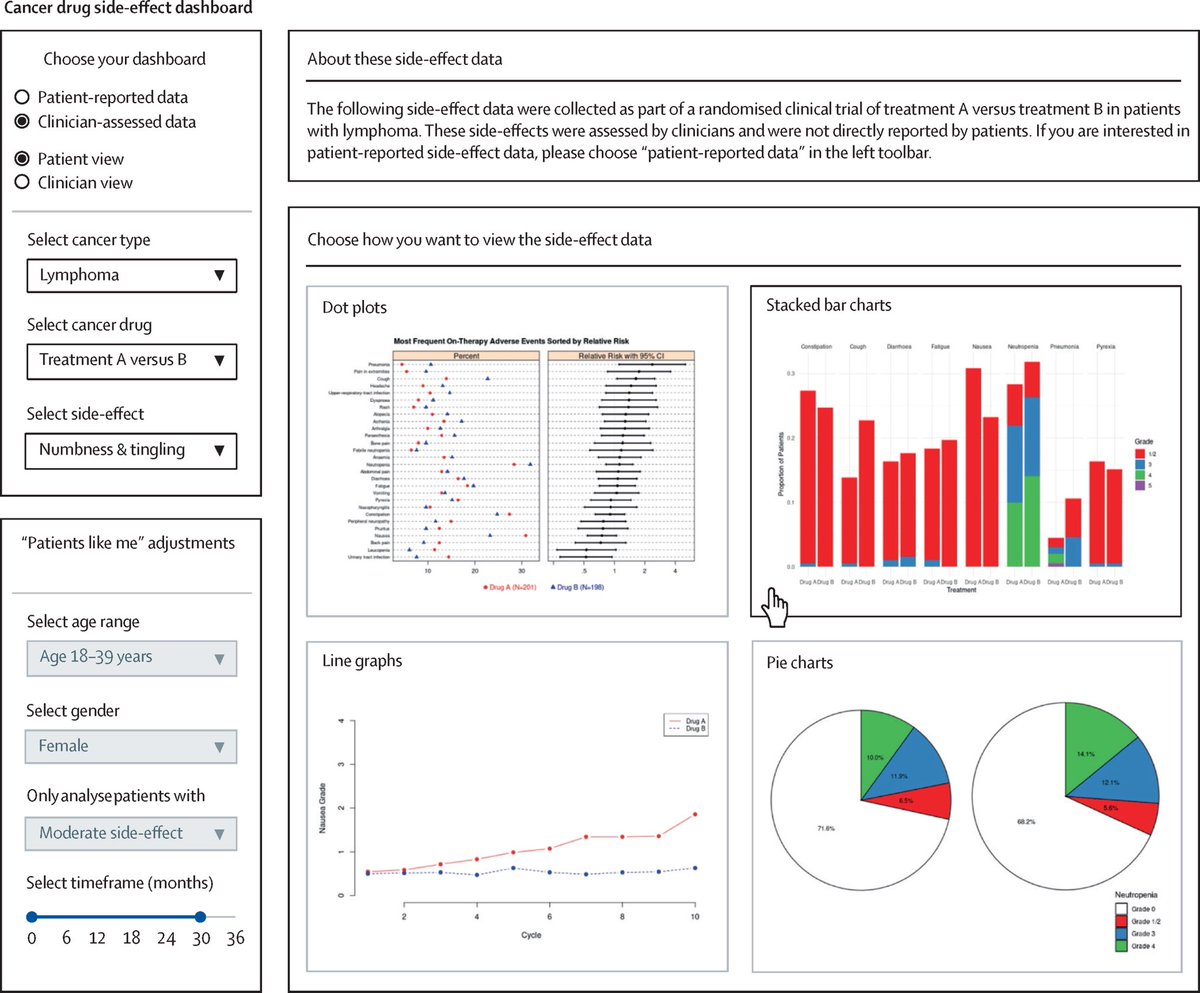
As blood cancer treatments evolve, so must the way we track side effects. #PROs are key to understanding tolerability, as explored in our paper led by Vishal Bhatnagar. 🩸Paper series led by @GitaThanaMD. #hematology #ClinicalTrials authors.elsevier.com/c/1lAZ98MsWYNW…

Excited to share our latest paper calling for a modernized approach to AE measurement, analysis, visualization, & reporting led by @majorajay! 📊🧬#hematology #ClinicalTrials #lymsm #dataviz authors.elsevier.com/c/1lAZ98MsWYNW…
Congrats NU Women’s Golf! Proud to be part of the Wildcat golf family! 💜
Northwestern defeats Stanford to capture their FIRST NATIONAL CHAMPIONSHIP in women's golf program history! 🏆👏 @NU_Sports 📺 Golf Channel | #NCAAGolf
Presenting a #patientreportedoutcomes workshop at Society for Clinical Trials 46th Annual Meeting! #SCT2025 #clinicaltrials #PROs @dpeipert

Remote Symptom Monitoring With Electronic Patient-Reported Outcomes in Clinical #Cancer Populations. jamanetwork.com/journals/jaman… @JAMA_current @JAMANetwork @JAMANetworkOpen #PallOnc #SuppOnc #CancerResearch
Hats off to Dr. Sumithra Mandrekar @MayoClinic - recipient of the 2025 Charles Moertel Lecture Award. Her lecture's entitled "Harnessing Alliance Clinical Trials Data to Drive Efficiency and Innovation in Cancer Trials Conduct and Research." #AlliancePlenary25 #GroupStatistician
An excellent #SuppOnc article re: #ePRO #ePRO can: ⬆️ communication 🗣️ 🤕symptom awareness 🧑💻 self-management. 🚧 Barriers exist. Needs better integration 💻, training,🎓 and actionable #pallonc content ✅ 🔗: ascopubs.org/doi/10.1200/OP… @EthanBasch1 @BiostatGirl @OncoAlert🚨…
Implementation of Symptom Monitoring With Electronic Patient-Reported Outcomes: Perspectives and Recommendations From Community Oncology Practices (Alliance AFT-39). ascopubs.org/doi/10.1200/OP… @ASCO @JCO_ASCO @JCOOP_ASCO #PallOnc #CancerCare @ALLIANCE_org @EthanBasch1 @BiostatGirl
Honored to be part of @ASH_hematology Clinical Research Training Institute & Training Day, Mediterranean, Middle East, and Northern Africa. I’m inspired by the growth of the trainees this week & I’m excited to see the future #hematology leaders they become! #CRTI #ASHCRTI



