
BAE_Lab
@Bae_Lab
Group of Prof. Hanyong Bae @ Dept. Chemistry, SKKU. Reaction Design & Catalysis Lab.
Check out our new paper in Cell Reports Physical Science, entitled "Pinacolborane-assisted superacid organocatalysis enabled direct access to cytotoxic alkyl N-Cbz amines". Congratulations to Woo Hee! cell.com/cell-reports-p…

Thrilled to share our collaborative work with Prof. Yoonsu Park’s group published in Nature Communications: Superacid counteranion as flexible-coordinating ligand for asymmetric organo-bismuth catalysis. Congratulations to Jin Hyun and Seok Yeol! doi.org/10.1038/s41467…

We are truly honored to have our recent contributions to "water-enhanced catalysis" featured in Accounts of Chemical Research, where we highlight its promise in expanding the landscape of modern catalysis pubs.acs.org/doi/10.1021/ac…

A new paper collaboration with Prof. Min Sang Kwon's group (SNU) in Cell Reports Physical Science, "Water-accelerated single-electron transfer aquaphotocatalysis for open-chain SuFEx hubs" is now published. Congratulations to Byeongjun and Yonghwan! cell.com/cell-reports-p…

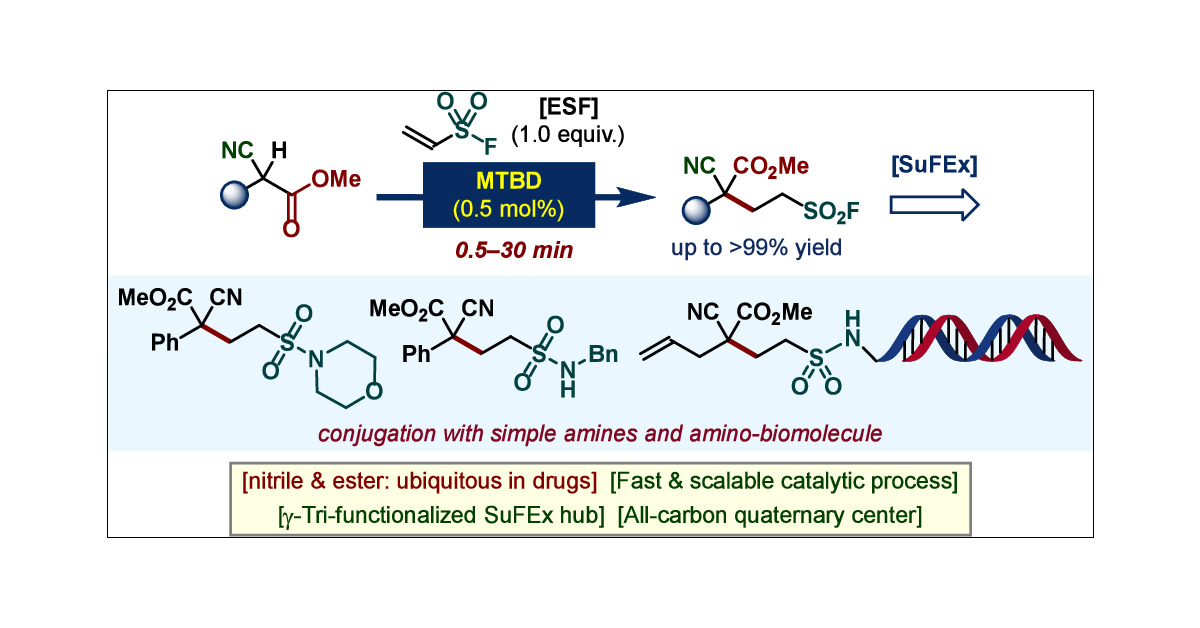
A new paper entitled "Highly Functionalized SuFEx-able Hub Bearing All-Carbon Quaternary Center via Rapid Brønsted Superbase Catalysis" from our group is now available in Organic Letters. Congratulations Soyeon!! pubs.acs.org/doi/abs/10.102…
Our new paper entitled "“On-Water” Accelerated Dearomative Cycloaddition via Aquaphotocatalysis" is now published in Nature Communications. Congratulations to Soo Bok! nature.com/articles/s4146…

Glad to share our first publication this year @ChemCatalysis, trityl tetrakis(pentafluorophenyl)borate as a sustainable precursor in silylium Lewis acid organocatalysis! sciencedirect.com/science/articl…
I finally visited my alma mater SKKU met old friends, mentors, and teachers after a long break. Thanks to @Bae_Lab for organizing the trip. Korea is certainly one of the most dynamic and vibrant countries which can contribute to the whole world in terms of R&D.
Out in Green Chemistry 📢 A new PPM-level organocatalyst for metal-free, solvent-free, and purification-free cyanosilylation of ketones! A work from @Bae_Lab 🎉 Read here 👇 doi.org/10.1039/D3GC00…
Our new paper entitled "Sustainable Oragnocatalytic Cyanosilylation of Ketones by PPM-Level Loading Triphenylcarbenium Tetrakis(pentafluorophenyl)borate" is now published in Green Chemistry. Congratulations Muhammad! pubs.rsc.org/en/content/art…

A new paper "Alkyl Sulfonyl Fluorides Incorporating Geminal Dithioesters as SuFEx Click Hubs via Water-Accelerated Organosuperbase Catalysis", a collaborative work with Prof. Paul Ha-Yeon Cheong group is published in Org. Lett. Congratulations to all! pubs.acs.org/doi/full/10.10…

Prof. Tsuji from Hokkaido University visited Bae Lab!!
Annyeong from Korea!
We are happy to announce that Han Yong is selected as this year's recipient of the Young Organic Chemist Award at KCS Organic Chemistry Division, together with Prof. Jun-Seok Lee @junseoklee! Congratulations a lot to Prof. Bae and Prof. Lee!



We at #NobelPrize symposium to honor Ben. It was great to welcome our alumni back in Mülheim @maxplanckpress
A bubbly welcome 🎉 #benlist2021 #nobelsymposium @NobelPrize @maxplanckpress
Wow...a night to remember, 💥💥 most awaited event of #MPIKOFO .. celebrating Nobel prize 2021 of Nobel laureate Prof. List. @ListLaboratory @JinsunLee11 @Bae_Lab #Nobel_Prize
Our group published a new paper in ChemSusChem, entitled "β-Aminosulfonyl Fluorides via Water-Accelerated N-Heterocyclic Carbene Catalysis". Congratulations to Jin Hyun!! …mistry-europe.onlinelibrary.wiley.com/doi/10.1002/cs…

Today, we published a new paper in ChemSusChem, entitled "Hydrophobic Amplification Enabled High-Turnover Phosphazene Superbase Catalysis". Many congratulations to Sun Bu!! …mistry-europe.onlinelibrary.wiley.com/doi/10.1002/cs…

Our group published a paper entitled "Efficient Access to General α-Tertiary Amines via Water-Accelerated Organocatalytic Multicomponent Allylation" in Nature Communications! Many congratulations on all members involved!! nature.com/articles/s4146…
Breakthrough for organocatalysis: a highly challenging reaction of a small unbiased substrate toward a pharmaceutically relevant product is accomplished with better than enzymatic selectivity. Our recent work @Nature by @huizhou91983288 nature.com/articles/s4158…