Wei Liu
@WeiLiuLab
Organic Chemist at University of Cincinnati
Now online: Article by Wei Liu @WeiLiuLab & co-workers @UCincinnatiChem Synthesis of chiral difluoromethyl cyclopropanes through desymmetric difluoromethylation of cyclopropenes enabled by enantioselective copper catalysis nature.com/articles/s4416… ($)
Our latest Al-Fe research is published in @J_A_C_S. Led by my student Roushan @ChemistryatUIC and our collaboration with Dan Ess of BYU, we are starting to map the chemistry of Al(II) through this mild approach! doi.org/10.1021/jacs.5…
Thrilled to share our newest work on the enantioconvergent synthesis of alkyl fluorides with KF, made possible by a synergistic combination of chiral urea and onium salt catalysts. Out today in @NatureCatalysis doi.org/10.1038/s41929… Congrats to @Claire_doo and all of the team!
Our group is looking for a PostDoc with a strong background in asymmetric organic synthesis. Come and join us at San Diego, it's beautiful and warm here.
I am thrilled to share our recent Nature Catalysis cover-featured paper on engineering nonheme iron enzymes for metallophotoredox catalysis, enabling decarboxylative C-N₃ and C-SCN bond formation nature.com/articles/s4192…. This work was pioneered and led by graduate student Jinyan…
We do Ni too!!! Please check this article published in @InorgChem in which our awesome undergrad student Daniel Ye (1st author!) describes the synthesis, characterization and PCET reactivity of a mononuclear NiOH system in three oxidation states. Enjoy! pubs.acs.org/doi/full/10.10…
Congrats to Margaret @mapb333 on her new publication in @angew_chem in collaboration with @SzymczakLab on the role of electron transfer in copper-promoted trifluoromethylation! @OSU_CBC onlinelibrary.wiley.com/doi/10.1002/an…
Our work on nonheme iron enzyme-catalyzed olefin trifluoromethylative difunctionalization is now published in @J_A_C_S ! (link: pubs.acs.org/doi/abs/10.102… Previous ChemRxiv version chemrxiv.org/engage/chemrxi…). This study was led by graduate students @zhangg_james & @ajhuls1 , with key…
POSTDOC AD: I'm looking to hire a postdoc who can bring their expertise in low-valent main group molecular synthesis and apply it to applications in molecular electronics. Sounds cool, right? More details and application instructions are attached. Please help spread the word!
Excited to share our latest work on chemical permutation, now published in @Nature! Using photochemical conditions, we've developed a method to selectively and predictably transform the structures of thiazoles, isothiazoles, and other azole systems.
Congrats to Gaoyuan, Arman, Sahil & the Musaev Lab on uncovering new reactivity with the CoH catalyst! Their work shows how ACT can drive allyl carboxylates beyond classic rearrangement pathways.🌟 Exploring their exciting findings here." pubs.acs.org/doi/10.1021/ja…
Overcoming Cu Reduction Limitation in Asymmetric Substitution: Aryl-Radical Enantioconvergent Cyanation of Alkyl Iodides | Journal of the American Chemical Society @uofcincy #Cu #Reduction #Asymmetric #Substitution #Enantioconvergent #Cyanation #Iodides pubs.acs.org/doi/10.1021/ja…
Did not expect to receive a crystal plaque for the promotion.😃@UCincinnatiChem

Check out our most recent work in @ChemicalScience. We report a photochemical approach where ligated amine-borane radicals serve as XAT mediators for the metal free, Sonogashira-like alkynylation of alkyl halides using a SOMOphilic approach⬇️ pubs.rsc.org/en/content/art…
Are you a #biochemist looking for a #tenuretrack #facultyjob? My colleague @GrilloAnthonyS and I are co-chairing the search & we are looking for an excellent colleague to join our growing and thriving department @UcChem @uofcincy @UC_ArtSci #Chemjobs tinyurl.com/2ukfwxt7
Excited to share the preprint of our latest work, led by graduate students James Zhang @zhangg_james and Anthony Huls @ajhuls1, on engineering nonheme iron enzymes for new-to-nature transformations! We evolved hydroxymandelate synthase from Amycolatopsis orientalis (AoHMS) to…
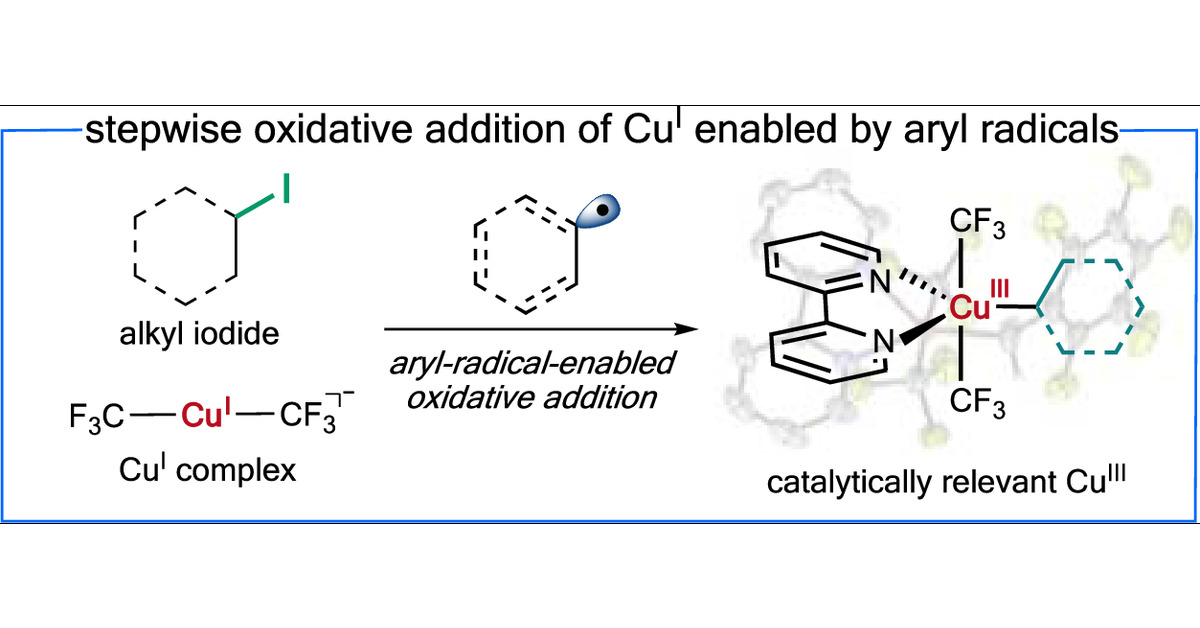
Catalytically Relevant Organocopper(III) Complexes Formed through Aryl-Radical-Enabled Oxidative Addition | Journal of the American Chemical Society @uofcincy @LifeAtPurdue @PurdueScience #Organocopper #ArylRadical #Oxidative #Addition #Catalysis pubs.acs.org/doi/10.1021/ja…
We have demonstrated @JACS the stepwise oxidative addition of Cu(I) to well-defined Cu(III) via an aryl-radical-enabled pathway, a step proposed in many Cu-catalyzed reactions. Huge thanks to @shiliangtian and @ZhangLabOSU for the EPR studies. pubs.acs.org/doi/10.1021/ja…
Why does nature use tricopper center to perform oxygen reduction? Check out our @J_A_C_S paper on bond dissociation energy, E1/2, pKa of tricopper clusters at all oxidation and protonation states! Congratulations to Saikat! @OSU_CBC #copper pubs.acs.org/doi/10.1021/ja…
Our work on electroreductive XEC from isolable Ni(aryl) complexes with @DipaKalyani1 is now online in @NatureChemistry! Great job everyone! nature.com/articles/s4155…