
Uppsala Monitoring Centre
@UMCGlobalSafety
Uppsala Monitoring Centre promotes the safer use of medicines and vaccines for everyone everywhere. We operate the WHO Programme for Int'l Drug Monitoring.
🎥 How is #VigiFlow shaping pharmacovigilance across the WHO PIDM? Manal Younus and Jaber Jaber share how they're utilising VigiFlow and connected applications to monitor the safety of medicines and vaccines in Iraq🇮🇶 and Jordan🇯🇴. Watch the film below for more👇
📖 Looking for a glossary of #pharmacovigilance terms and definitions? Version 2.3 of the CIOMS Cumulative Glossary compiles all terms and definitions included in CIOMS Working Group reports on pharmacovigilance and related topics. Check it out below👇 cioms.ch/publications/p…
🎥 How can data-driven semantic vector representations help analyse #pharmacovigilance data? Joana Félix sat down in the Research Corner to explain how vigiVec can produce stable and clinically related vector representations for adverse events and drugs. Watch the film below👇
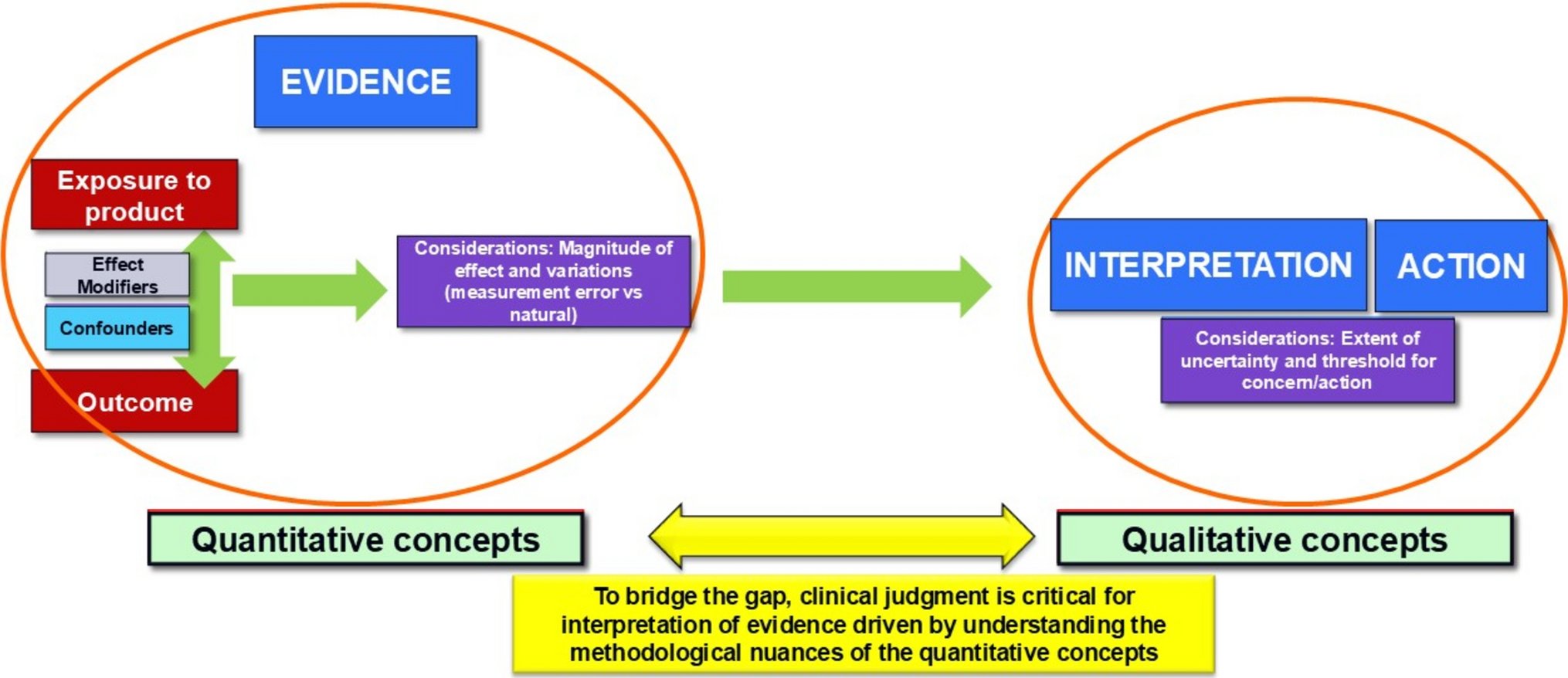
📚How can we bridge different perspectives when it comes to decision-making in #DrugSafety? This paper provides a framework that aims to assist PV stakeholders in navigating these challenges and improving regulatory & clinical decision-making processes👇 link.springer.com/article/10.100…
🎥 𝙒𝙝𝙖𝙩 𝙙𝙤𝙚𝙨 #𝙈𝙚𝙙𝙎𝙖𝙛𝙚𝙩𝙮𝙒𝙚𝙚𝙠 𝙢𝙚𝙖𝙣 𝙩𝙤 𝙮𝙤𝙪? Since 2016, we've teamed up with #pharmacovigilance stakeholders worldwide for the #MedSafetyWeek campaign. @Armidaze, Head of @COFEPRIS, shares Mexico🇲🇽's Med Safety Story in the film below👇
We hope you've enjoyed following #MedicationErrorsFridays as we've explored the critical stages, vulnerable populations, & other areas where errors can occur, such as #MedicalDevices. Be sure to read #UppsalaReports for more on #MedicationErrors 👉 ow.ly/F4q150W5qYF
📨 A new #CIOMS Newsletter is available! This edition features new publications on severe cutaneous reactions, benefit-risk balance, CIOMS working group news, international events, and regulatory updates. Read it today 👉 ow.ly/9LVX50WlmBh

Side effects don't take vacation 🏖️ If you find yourself by the water this summer, report side effects to your healthcare professional. Enjoy the sun safely, like our blobs from #MedSafetyWeek 2020 & 2023. Learn more 👉 ow.ly/XEoc50LlhaJ
📩 Our #UppsalaReports newsletter brings the latest updates from the world of #pharmacovigilance straight to your inbox, including trending tools and technology, new research methods, and PV-related events around the globe. Subscribe today 👉 uppsalareports.org/subscribe

What is #pharmacovigilance? Reports of potential side effects from patients and healthcare professionals help us improve patient safety worldwide. 👀 Find out how the #MedSafetyCycle works in this illustrated story 👉 ow.ly/EoZ950LOJE4
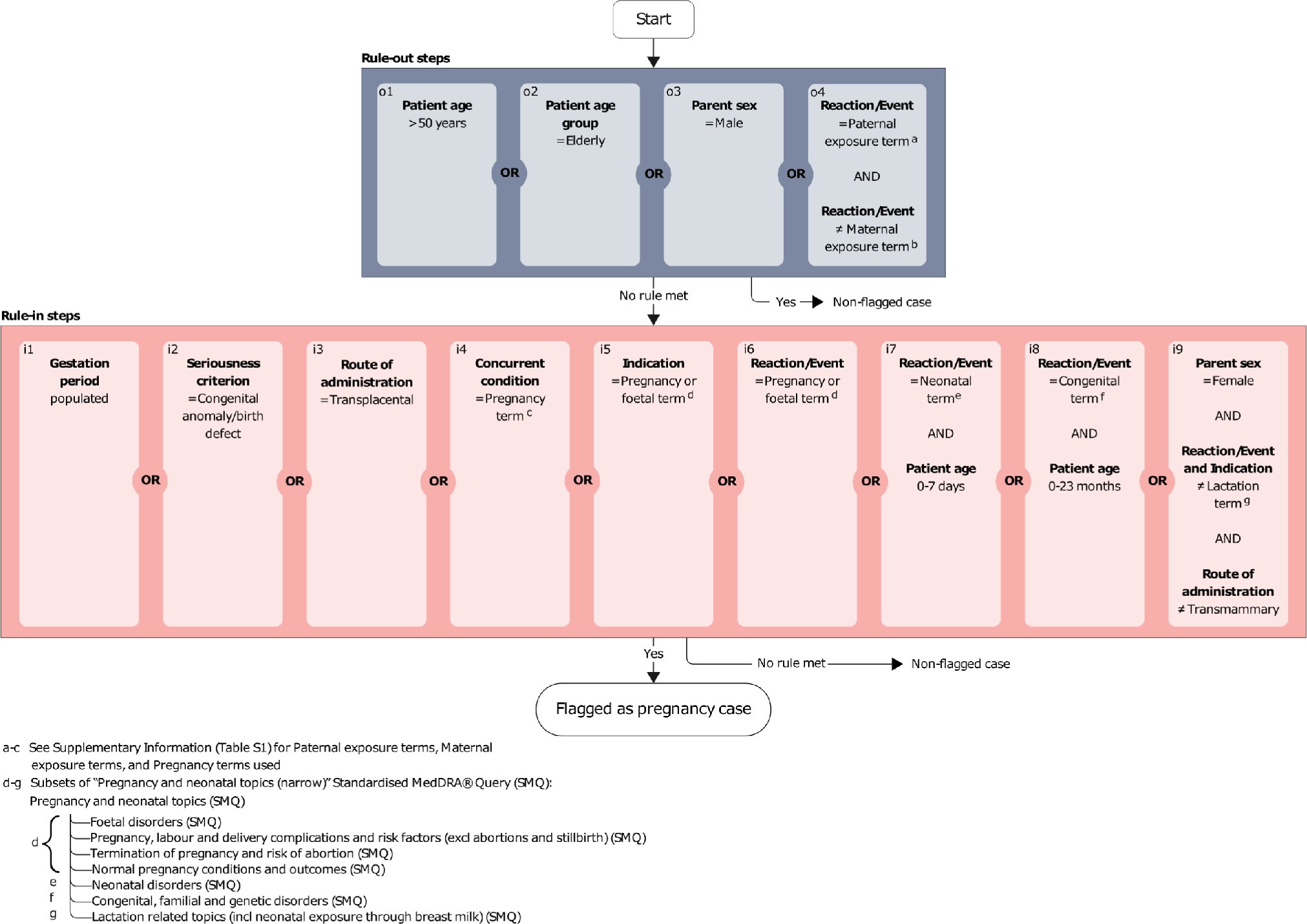
One challenge of #pharmacovigilance is retrieving case reports involving pregnancy. UMC researchers demonstrate the robustness of an algorithm in uncovering pregnancy-related cases in #VigiBase in this #DrugSafety article. 📖 #OpenAccess below👇 doi.org/10.1007/s40264…
📅 Save the Date – 9th ISoP Seminar on: Intelligent Automation in Pharmacovigilance 🗓️ 4–5 December 2025 📍 Boston–Cambridge, USA 📌 📧 Register your interest by emailing: [email protected] #ISoPOnline #ISoPSeminar #Pharmacovigilance #DrugSafety #Automation
Stay informed about all things #pharmacovigilance by listening to our podcast #DrugSafetyMatters. Some of our latest episodes cover: 📝 Quality in ADR reporting 🫄 Pregnancy-related pharmacovigilance 🐾 Veterinary pharmacovigilance 🎧 Listen now at 👉 drugsafetymatterspod.org