TGA Australia
@TGAgovau
Australia's medicines and medical devices regulator See our website for our social media acceptable use policy.
⚠️Recall – SpringLeaf Fat Clear⚠️ Homart Pharmaceuticals Pty Ltd is recalling specific batches of its product, SpringLeaf Fat Clear, due to two identified compliance problems. Read more: tga.gov.au/safety/market-…

⚠️Medicine shortages update - variable supply of methylphenidate products extended⚠️ Shortages for Concerta modified-release tablets have been extended until the end of 2026. There will be periods when supply is limited or some strengths are unavailable: tga.gov.au/safety/shortag…

We have facilitated the seizure of millions of counterfeit and illegal therapeutic goods as part of INTERPOL's Operation Pangea XVII, a global initiative targeting the illegal trade of therapeutic goods. Read more: tga.gov.au/news/media-rel…

⚠️ Safety advisory- Counterfeit Botox vials detected ⚠️ Two unrelated cases of counterfeit injectable botulinum toxin products have been stopped at the Australian border. The counterfeit products were packaged to appear as genuine, branded, Botox products. tga.gov.au/news/safety-al…

⚠️Recall – Xinyi Biyan Pills ⚠️ Well Herb is recalling batch numbers: DB0014M & DB0015M with expiry Date 11/12/2027 supplied in Australia of Xinyi Biyan Pills due to the medicine having unacceptable amounts of lead above the permitted daily dose. tga.gov.au/safety/market-…

We have conducted a review of sunscreen ingredients used in Australia and is recommending additional safeguards for 2 ingredients and a sunscreen ingredient by-product (degradant). Read more: tga.gov.au/news/media-rel…

⚠️Update on Product Correction A40/30 BiPAP Series⚠️ Due to ongoing problems with A30 & A40 series bi-level positive airway pressure devices, Philips is contacting affected customers & patients to organise replacement devices or discuss alternatives: tga.gov.au/news/safety-al…

See the latest compliance review results for 51 listed medicines, including products containing valerian, and products making non-permitted claims about anxiety, bone strength, cold sores, muscle performance, and sunscreen coverage 👉 tga.gov.au/resources/list…

Australians who rely on medical devices will benefit from new measures introduced by the Australian Government to enhance the identification and management of device-related safety concerns. Read more: tga.gov.au/news/media-rel…

⚠️Medicine shortage update - Discontinuation of Mounjaro (tirzepatide) vials⚠️ Eli Lilly has informed us that all strengths of Mounjaro vials are now discontinued due to commercial reasons and supply of all vial presentations has been exhausted: tga.gov.au/safety/shortag…

The TGA is aware that recent media reports have stated that the TGA estimates that more than 10 million vapes are sold nationally every month on the black market. These reports are incorrect. Read more: tga.gov.au/news/media-rel…
From 1 July 2025, restrictions under IChEMS will prohibit the import, manufacture and use of three PFAS chemicals in Australia. IChEMS does not apply to chemicals used for therapeutic purposes. This means medical devices will not be affected by the ban: tga.gov.au/news/news/medi…

We have accepted a court-enforceable undertaking from The Aussie Gelatin Company Pty Ltd in relation to the alleged unlawful manufacture, supply and advertising of therapeutic goods. Read more: tga.gov.au/news/media-rel…

⚠️Safety advisory - Counterfeit Rybelsus Semaglutide tablets detected⚠️ Examples of counterfeit Rybelsus-labelled products have been stopped at the Australian border. These tablets may pose a serious risk to your health and should not be used. Read more: tga.gov.au/news/safety-al…

⚠️Safety advisory - Iverjohn-12 & Ivervid-12 tablets⚠️ We're continuing to see imports of counterfeit ivermectin. Laboratory analysis confirmed Iverjohn-12 & Ivervid-12 contain less than the stated 12mg of the active pharmaceutical ingredient, ivermectin: tga.gov.au/news/safety-al…

⚠️Safety advisory - Counterfeit ‘Laroscorbine Platinum’ branded product detected⚠️ Consumers and health professionals should be aware that examples of counterfeit Roche labelled products have been stopped at the Australian border. Read more: tga.gov.au/news/safety-al…

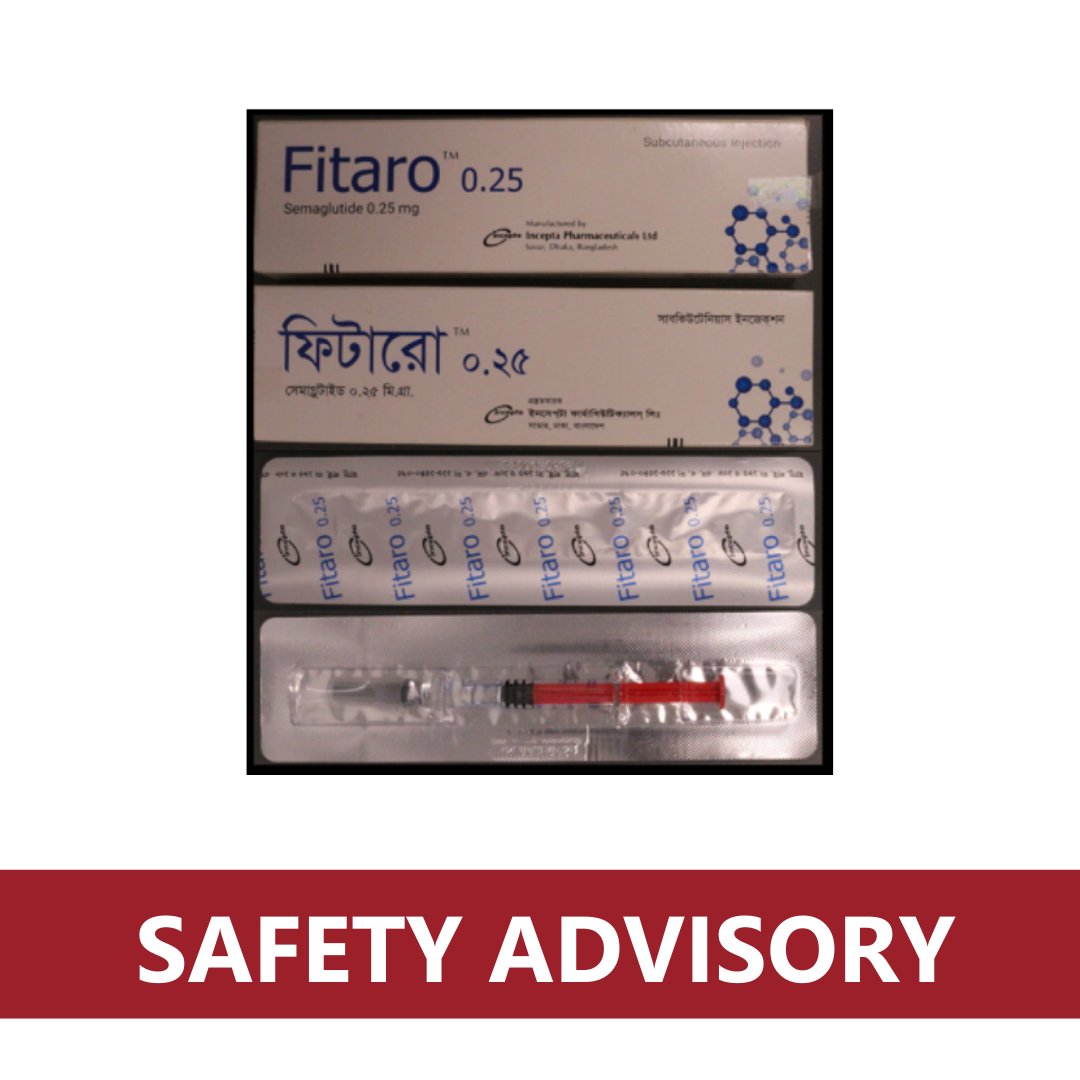
⚠️Safety advisory - Fitaro semaglutide pre-filled syringe (various dosage strengths)⚠️ Consumers and health professionals should be aware that examples of substandard Fitaro semaglutide pre-filled syringes have been stopped at the Australian border: tga.gov.au/news/safety-al…
⚠️Medicine shortage update - Methylphenidate products shortage: clinical guidance available⚠️ The Australasian ADHD Professionals Association, a member of our MSAG, has developed guidelines to help clinicians manage the ADHD medicine shortages. More: tga.gov.au/safety/shortag…

⚠️Recall – HeartSine Samaritan Public Access Defibrillators (PAD) 350P, 360P, 500P⚠️ Stryker is conducting an Urgent Recall of certain serial numbers of the HeartSine Defibrillators due to a manufacturing problem relating to a circuit board component: tga.gov.au/safety/market-…

We have issued an infringement notice of $18,780 to Victorian-based company, Nourishmeorganics Pty Ltd, for the alleged unlawful supply of a therapeutic good not entered in the Australian Register of Therapeutic Goods (ARTG). Read more: tga.gov.au/news/media-rel…
