Matthew Gaunt
@MatthewGaunt8
The @Gaunt_Group are looking for two enthusiastic and talented researchers to join our dynamic team in Cambridge, as postdocs. If you are interested in developing cutting edge synthetic chemistry across a wide range of areas relating to catalysis, apply at jobs.cam.ac.uk/job/49113/
3-Selective Pyridine Fluorination via Zincke Imine Intermediates | Journal of the American Chemical Society @ColoradoStateU @TotalSyntheses pubs.acs.org/doi/10.1021/ja…
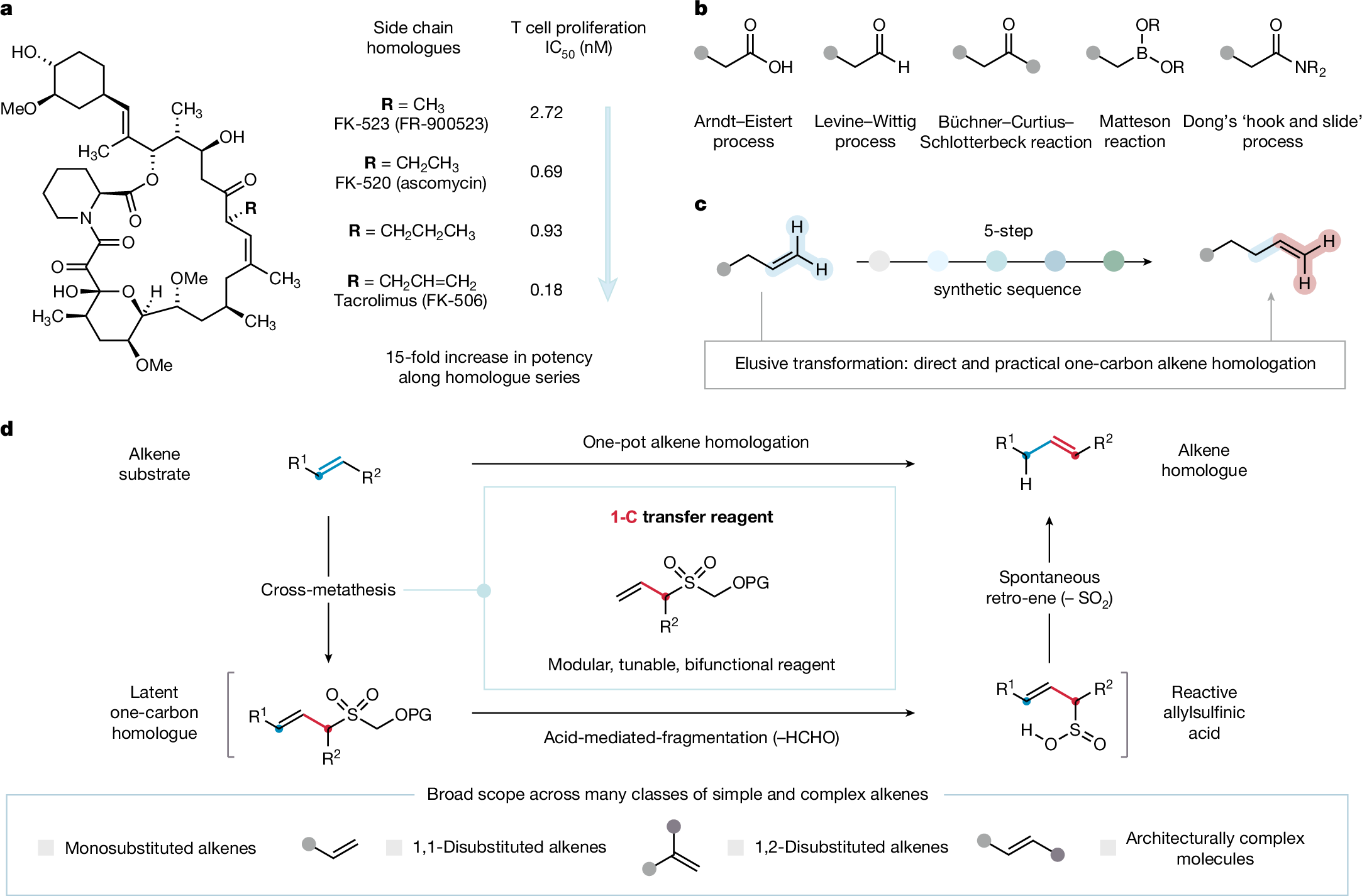
We are excited to share our new work on one carbon alkene homologation. We can iteratively homologate terminal and internal alkenes in simple and complex molecules using this method. Great work by Marcus that opens up lots of exciting opportunities for us. nature.com/articles/s4158…
Congratulations Manuel
Very happy to share that our last work: "Photocatalytic deoxygenative Z-selective olefination of aliphatic alcohols" has found home in @NatureComms!!! Many congrats to @sainarayanan89, @irenessordo, @CarlaAiraRodrgz and @ManuAzzi for the amazing work! rdcu.be/ef9VO
Happy to see our Carbonyl Azinylative Amination work by Alex, Roopender and Rachel forming diverse heteroarylaklyamines using in situ generated heteroarylindium species highlighted in @ACSPublications OPR&Ds new items of interest! pubs.acs.org/doi/10.1021/ac…
Very excited to share our latest protocol in @NatureCatalysis for photocatalytic generation of alkyl carbanions - no need organometallic reagents or reducing metals. Starting from alkene feedstocks, it works with diverse carbon electrophiles. 💥 A Herculean effort led by…
Amazing paper from the group of my Cambridge colleague Robert Phipps. Great work. science.org/stoken/author-…
Come and join our team. We have an exciting Synthetic Chemistry PhD opportunity in the @Gaunt_Group (starting Oct 2025) in collaboration with AstraZeneca. It will focus development of new catalytic activation modes for the synthesis of novel scaffolds. jobs.cam.ac.uk/job/48481/
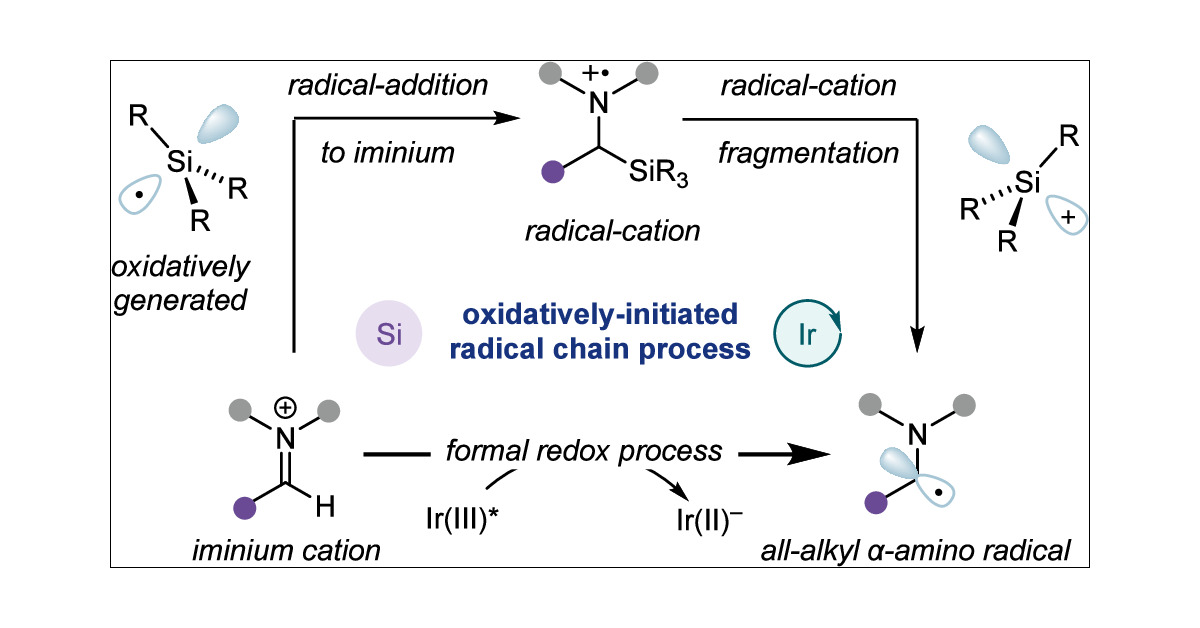
New from our photocatalytic multicomponent amine synthesis program. A mechanistically distinct method for the generation of unbiased a-amino radicals formed from iminium reduction. Great work by Harry, who solved some challenging problems in this project. pubs.acs.org/doi/10.1021/ja…
Here’s a straightforward and practical way to make heteroaryl substituted alkyl amines. Surprising how few methods generate this type of useful functionality. Great work by Alex (now at Dr Reddy’s), @Roopender_Kumar (Assist. Prof at UCL) and Rachel (starting final year of PhD).
Modular Synthesis of Heterobenzylic Amines via Carbonyl Azinylative Amination (Matthew J. Gaunt and co-workers) @Gaunt_Group #openaccess onlinelibrary.wiley.com/doi/10.1002/an…
Nice work Manuel and team
Very happy to share our last work: "Deoxygenative Z-selective olefination of aliphatic alcohols", now in @ChemRxiv 🎉! Congrats Sai (@sainarayanan89 ), Irene (@irenessordo), Carla (@CarlaAiraRodrgz) and Emanuele (@ManuAzzi), amazing work! Check it out 👇 chemrxiv.org/engage/chemrxi…
I will start my independent career at Texas Tech @TexasTech in January 2025! (whitehurstlab.org) Thanks to my mentors @MatthewGaunt8 @pchirik @ritter_lab!
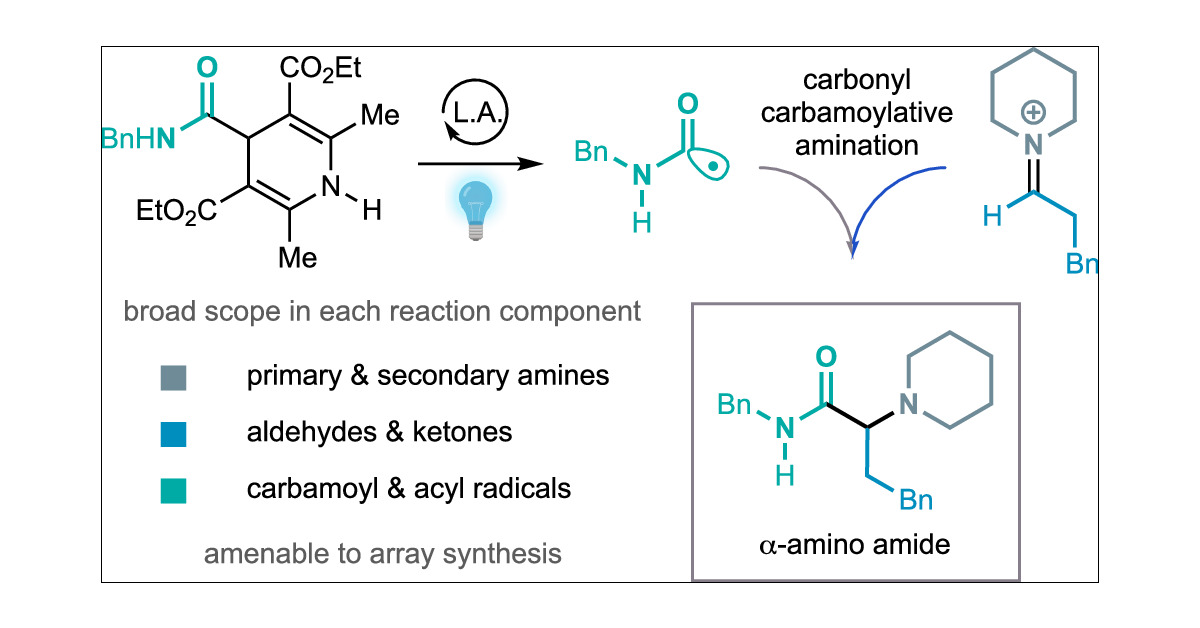
Here’s another contribution to our amine synthesis platform. A modular, practical and general carbonyl acylative amination to alpha-amino amides and ketones. Great work by Jianzhong Liu. He’s looking for academic positions and is definitely one to watch. pubs.acs.org/doi/10.1021/ja…
Here’s a new paper from our amine synthesis program. pubs.rsc.org/en/content/art… Led by @miloasmith and supported by Ryan Kang and @Roopender_Kumar, this works provides a mild way to make branched 2’-alkylamines Follow @miloasmith as he moves onto his next adventure with @Shenvi_Lab
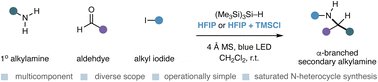
Our Carbonyl Azinylative amination (CAzA) for the synthesis of α-heteroaryl amines is now out in @angew_chem! Congrats Alex (@alex_rafaniello), Roopender (@TheKumarLab1) and Rachel!🎉 Read it here 👇 onlinelibrary.wiley.com/doi/10.1002/an…
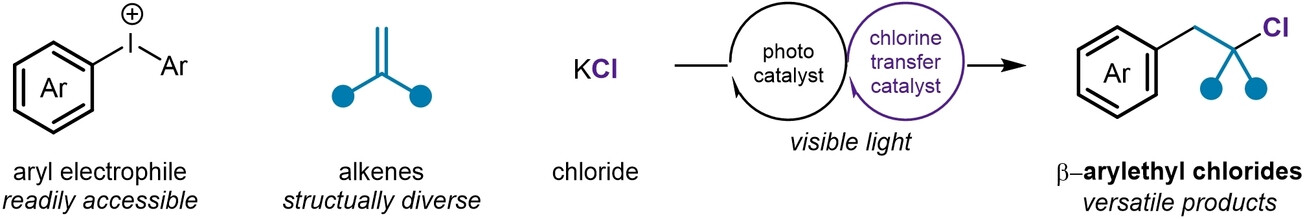
A modular dual catalyst system enables tuneable alkene chloro-arylation to highly functionalized chloroalkanes. Congrats to Bo, Ala, Daniel, Katherine and Jean. Out now in @angew_chem onlinelibrary.wiley.com/doi/10.1002/an…
This might look like a reaction you think is well established, but it’s not been possible before now. Here’s a modular and practical process to make these molecules in a single step. Great work by Alex, @Roopender_Kumar and Rachel that’s finally online. onlinelibrary.wiley.com/doi/10.1002/an…

Great news: our group's first paper is out in ACIE! Thanks a lot to the team and our collaborators @kirchner_group , Schiemann, Kielb Groups. It was an incredible journey! A General Iron‐Catalyzed Decarboxylative Oxygenation of Aliphatic Carboxylic Acids onlinelibrary.wiley.com/doi/10.1002/an…
Here is our work on pyrimidine diversification with @bobbypaton. This approach effectively allows you to exploit de novo heterocycle synthesis at the later stages of drug and agrochemical development. Congrats to Ben, Celena, and Louis! nature.com/articles/s4158…
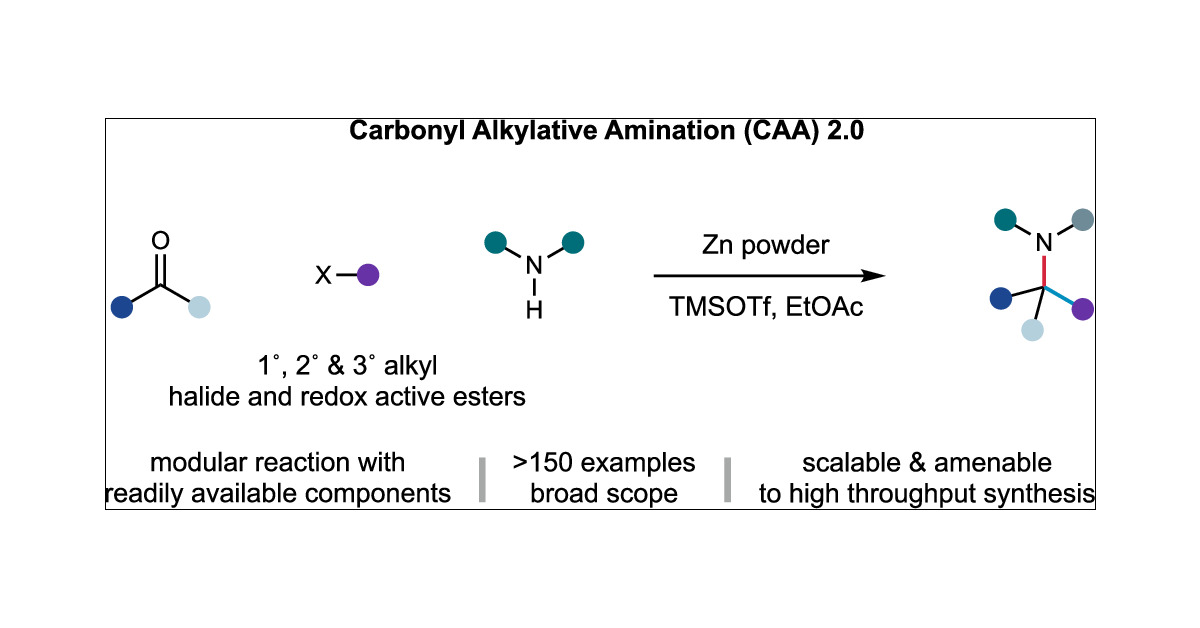
congratulations to the Joseph, @Roopender_Kumar, John, James, Nils and Sarah for their work on a broad amine synthesis platform. A pre-Covid project, this work is new take on an classic but under-appreciated problem. A lot of problems are solved here. pubs.acs.org/doi/10.1021/ja…