
Davide Capodanno
@DFCapodanno
Professor of Cardiology at University of Catania, Italy. Editor-in-Chief of @EuroInterventio.
After a few years, I’ve developed a clearer sense of the types of speaking invitations I enjoy—and those I don’t. 1) “I’m inviting you to my event—pick whatever title you like.” There’s nothing inherently wrong with this, and perhaps it’s even meant as a courtesy, but I don’t…

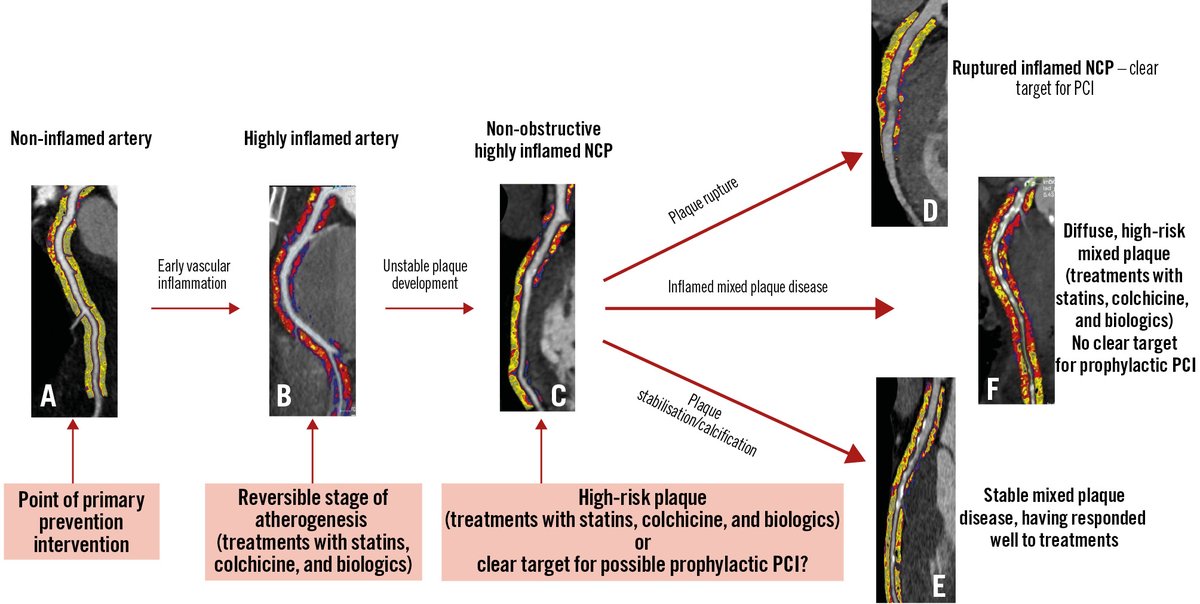
This State-of-the-Art review aims to facilitate a comprehensive assessment of plaque pathology using coronary imaging. It outlines how imaging characterizes plaque components and phenotypes, links plaque features to clinical events, and summarizes trials using serial imaging to…
CORCINCH-HF (NCT04331769) is a prospective, randomized, open-label, international, multicenter clinical trial designed to evaluate the safety and efficacy of the AccuCinch Ventricular Restoration System in patients with heart failure with reduced ejection fraction (HFrEF),…

Artificial intelligence (AI) has emerging applications in cardiovascular pharmacotherapy, including treatment selection, prediction of outcomes, and trial optimization. Our manuscript reviews current AI uses in therapy, trials, and drug discovery. academic.oup.com/eurheartj/adva…
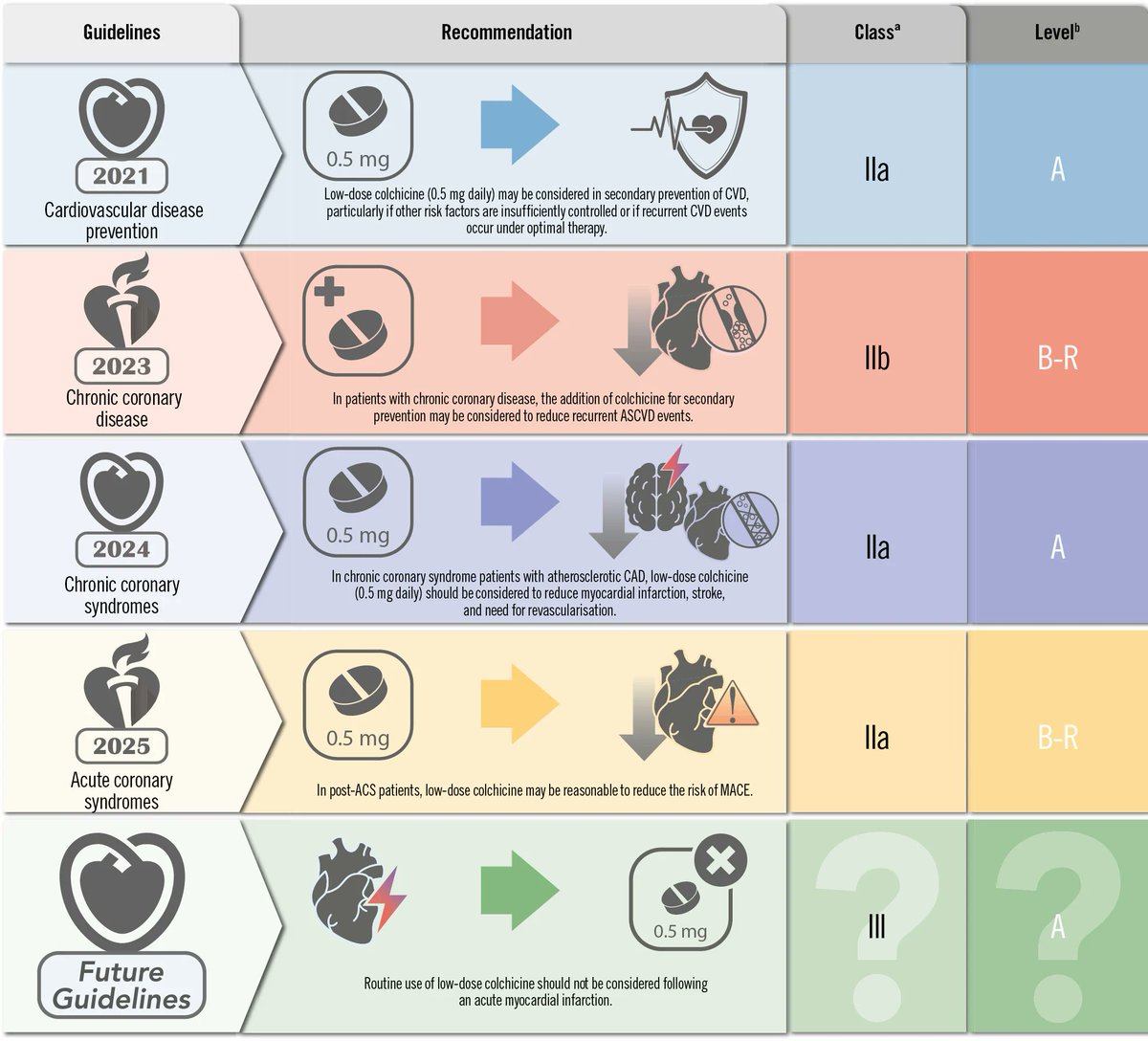
The role of inflammation in atherosclerotic cardiovascular disease is well established, and colchicine has emerged as the first anti-inflammatory agent recommended for ACS and CCS. The CLEAR trial, which recently showed neutral results in STEMI patients, has raised questions…
To all enthusiasts of platelets, hemostasis, and thrombosis: ESC Hot Line Session 7, focused on antiplatelet therapy in ACS/PCI, is notable for the coincidental fact that all featured trials had their protocol designs published in EuroIntervention. This is gratifying, as it…

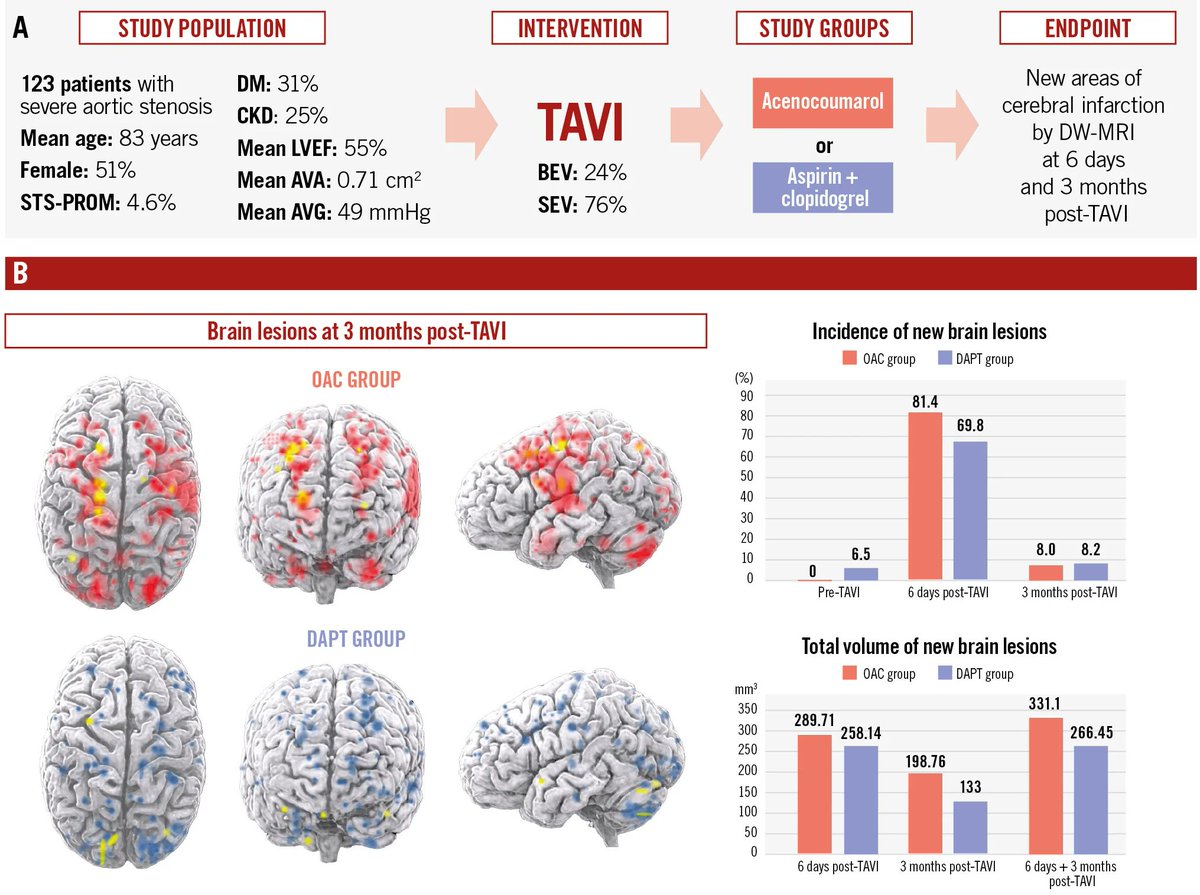
In the randomized, open-label AUREA trial, an OAC strategy for 3 months post-TAVI did not show any benefit over an antiplatelet strategy in preventing cerebral microembolism in patients without an indication for OAC. Patients treated with DAPT showed a lower mean volume of brain…
I am pleased to announce that the new Impact Factor of EuroIntervention is the highest in its 20-year history: 9.5. Ranked 16th out of 230 journals in the cardiovascular category (+5 positions versus the previous year). Thank you all — it’s an exciting rollercoaster.

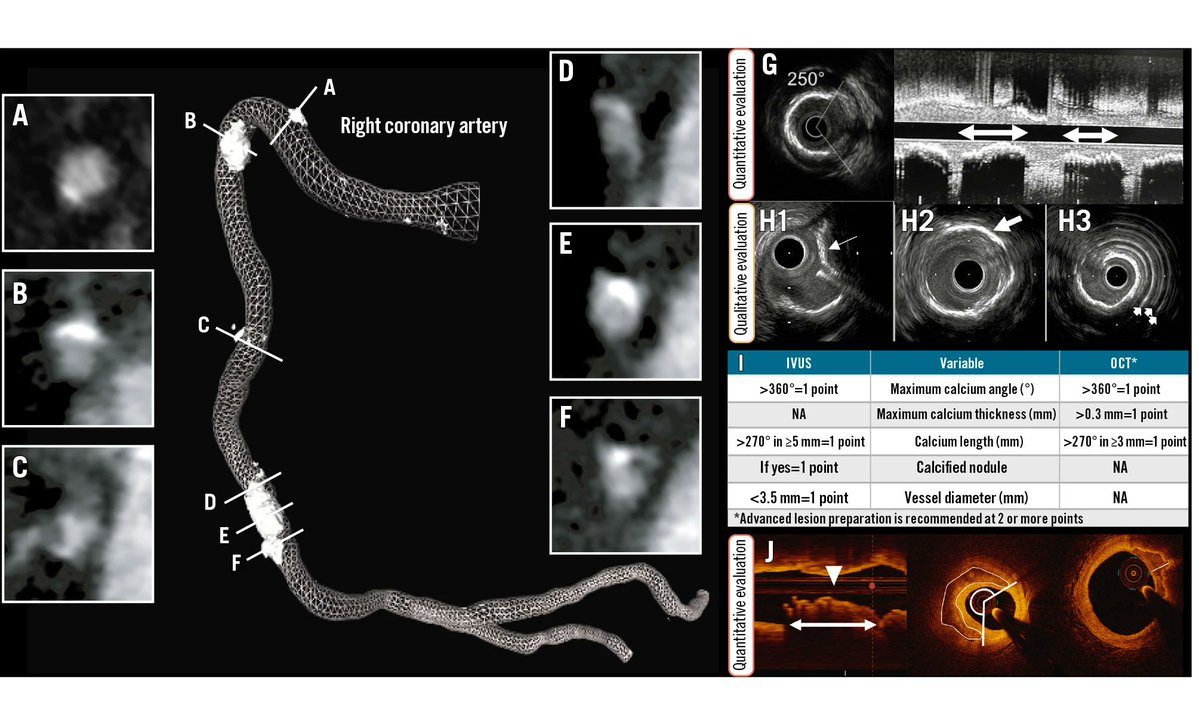
Calcified lesions represent one of the most challenging subsets for PCI. Successful treatment is feasible in selected cases through careful planning, data integration, and expert use of available devices. Below is an algorithmic approach to the use of balloons, atherectomy, and…
This newly proposed implanter classification from the European Left Atrial Appendage Closure Club (ELAACC) provides a structured framework to assess anatomical and functional challenges in left atrial appendage closure. eurointervention.pcronline.com/article/a-new-…

Coronary CT or invasive angiography as the first-line test in patients with stable angina? A clear question with an uncertain answer—though the guidelines do offer some direction.
Pericoronary adipose tissue (PCAT) attenuation on CCTA is a novel marker linked to increased cardiac mortality and major events. This study shows that PCAT attenuation independently predicts adverse outcomes after PCI and improves risk stratification when added to standard CCTA…
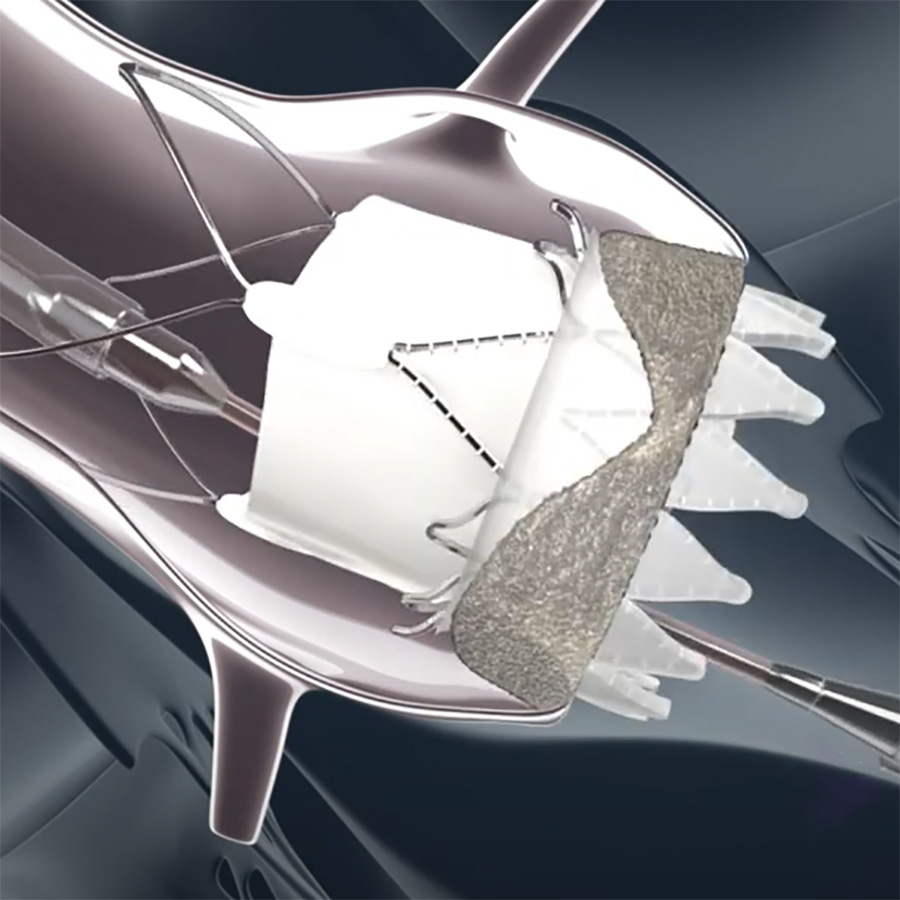
Coronary obstruction is a rare but serious complication during transcatheter aortic valve implantation (TAVI), particularly in valve-in-valve procedures, which is associated with high mortality. This review explores various leaflet modification techniques designed to mitigate…

In the field of interventional cardiology, today’s news is that Boston Scientific Corporation has announced the discontinuation of global sales of its ACURATE neo2™ and ACURATE Prime™ aortic valve systems. Over the years, various iterations of this valve have been evaluated in…
I’m always eager for updates on antiplatelet agents, so I thought it was worth sharing that CeleBrate, the pivotal Phase 3 trial of the subcutaneous GPIIb/IIIa inhibitor zalunfiban, has been completed. Results are expected in Q3 2025—possibly at AHA? The drug is being developed…
