NIH Pragmatic Trials Collaboratory
@Collaboratory1
Supported by @NIH, the NIH Pragmatic Trials Collaboratory is advancing the design, conduct, and dissemination of pragmatic clinical trials.
📣 In this Friday's Rethinking Clinical Trials Grand Rounds, Jon Tilburt and Andrea Cheville of @MayoClinic will present initial outcomes from NOHARM, an NIH Collaboratory Trial. Join us! #pctGR 🔗 duke.is/pctnews20250723

Decisions about whether to integrate digital tools into the EHR for #pragmatictrials hinge on 4 critical factors: complexity, institutional governance, requirements for integration, and involvement of personnel. Learn more: lnkd.in/ebyYi8dz

In this Friday's session of our Rethinking Clinical Trials Grand Rounds webinar, @DukeMedSchool's Adrian Hernandez and Rebecca Sullenger will lead a panel discussion on a recent analysis of ClinicalTrials.gov and the state of clinical trials. ➡️ duke.is/pctnews20250715

Features of the external environment during online medical group visits and interacting with the technology itself emerged as key themes in interviews with patients participating in OPTIMUM, an NIH Collaboratory Trial. Learn more ➡️ duke.is/pctnews20250716

Engaging with community partners can enrich and inform research through the lifespan of a clinical trial. NIH Collaboratory investigators shared tips for building these relationships at our 2025 Annual Steering Committee Meeting. Learn more ➡️ duke.is/pctnews20250714

Wonderful quote: “…one of the problems …is that we may think that our customers are grant review panels or maybe…editors,” …“Those may be our short-term customers, but …not … ultimate customers. Our ultimate customers are people who have to make decisions about healthcare”
🎙️ In the latest episode of our Rethinking Clinical Trials Podcast, we asked Rich Platt, Greg Simon, and Hayden Bosworth to discuss their recent @JAMA_current Viewpoint, "Making Pragmatic Clinical Trials More Pragmatic." ➡️ duke.is/pctnews20250626
At our 2025 Annual Steering Committee Meeting, panelists discussed the usefulness of the P value in healthcare systems research. #biostatistics Read more ➡️ duke.is/pctnews20250710

📣 In this Friday's Rethinking Clinical Trials Grand Rounds, Tyler Cope and @TrevorLentzPT of @DukeMedSchool discuss novel approaches to recruiting clinical sites in the AIM-Back pragmatic trial. Join us! #pctGR 🔗 duke.is/pctnews20250709

In an analysis of data for 123,000 seriously ill older adults by our PRIM-ER team, poor prognosis was associated with fewer healthy days at home. However, a cancer diagnosis was associated with more healthy days at home. Learn more ➡️ duke.is/pctnews20250708

In the OPTIMUM trial, trust in clinicians, positive interactions with study staff, and opportunities for social interaction were key to recruitment, retention, and engagement for first-time participants and those from underrepresented groups. Read more ➡️ duke.is/pctnews20250703

Embedding #pragmatictrials into #primarycare settings comes with special challenges. NIH Collaboratory investigators shared their experiences and advice at our 2025 Annual Steering Committee Meeting. Read more ➡️ duke.is/pctnews20250702

At our 2025 Annual Steering Committee Meeting, investigators discussed their recently completed NIH Collaboratory Trials and their thoughts about future directions for pragmatic research. Read more ➡️ duke.is/pctnews20250701

🎙️ In the latest episode of our Rethinking Clinical Trials Podcast, we asked Rich Platt, Greg Simon, and Hayden Bosworth to discuss their recent @JAMA_current Viewpoint, "Making Pragmatic Clinical Trials More Pragmatic." ➡️ duke.is/pctnews20250626

📣 In this Friday's Rethinking Clinical Trials Grand Rounds, we welcome Amrita Mukhopadhyay of @nyugrossman to discuss the BETTER CARE-HF pragmatic trial. Join us! #pctGR #cardiotwitter 📷 duke.is/pctnews20250625

🎉 The study design paper for ARBOR-Telehealth has been published. The study will test a telehealth physical therapy strategy for primary care patients with low back pain in rural areas. Congratulations to the study team on reaching this milestone! ➡️ duke.is/pctnews20250624

📚 We've got content! In a new section of our Living Textbook of Pragmatic Clinical Trials, we offer tips on how to choose patient-reported outcome measures in pragmatic trials. Learn more ➡️ duke.is/pctnews20250623

📣 In this Friday's PCT Grand Rounds, Scott Garrison of @UAlberta_FoMD and Canada's Pragmatic Trials Collaborative will present the BedMed and BedMed-Frail randomized trials. Join us! #pctGR #CardioTwitter 🔗 duke.is/pctnews20250618

📣 In this Friday's PCT Grand Rounds, Stephanie Morain, Nancy Kass, and Ruth Faden of the @JohnsHopkins Berman Institute will discuss improving the ethical oversight of pragmatic clinical trials. Join us! #pctGR 🔗 duke.is/pctnews20250611

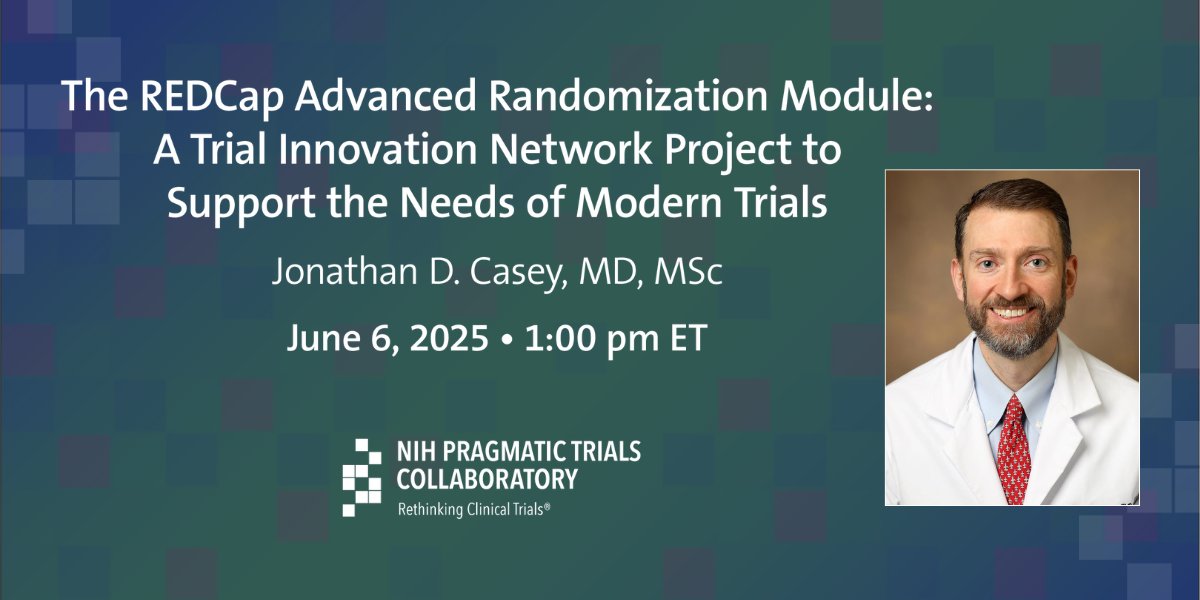
📣 In this Friday's PCT Grand Rounds, @JonathanCaseyMD of @PCCRG at @VUMChealth will discuss a REDCap advanced randomization module from the Trial Innovation Network. Join us! #pctGR 🔗 duke.is/pctnews20250604