
Adagene
@AdageneGlobal
Redefining the design and discovery of therapeutic antibodies #LifeIsMotion
This year marks our 10th anniversary – a decade focused on understanding the dynamic nature of antibodies to discover and develop transformative therapies for cancer. #CancerResearch #Adagene
Thanks @sitcancer for selecting our clinical abstract as a ‘Top 100’ out of 1439 regular abstracts at this year’s annual conference! Thanks to Aurelien Marabelle, Peter Luo, Jiping Zha and Songmao Zheng for hosting the updates onsite and all the authors of our two posters!


Excited to showcase data on masked anti-CTLA-4 SAFEbody, ADG126 (muzastotug) at SITC on Saturday, 11/9! Join us at poster #744 Saturday, 6:45 pm for a “Meet the Experts” session with Aurelien Marabelle, Peter Luo, Jiping Zha and Songmao Zheng. loom.ly/YxQuPlg

Thank you to Dr. Peter Luo, Dr. Daneng Li, Dr. Jiping Zha & Dr. Stan Frankel for presenting at #ESMO2024! We're grateful to the authors, sites, and everyone supporting our ADG126 + pembrolizumab study, and to all at ESMO advancing cancer care. #Immunotherapy #Oncology

Adagene announces positive FDA feedback on its clinical development plan for Muzastotug (ADG126) in MSS colorectal cancer following a productive Type B meeting. 🔗 Read more: investor.adagene.com/news-releases/…

We’re partnering with ConjugateBio to develop novel bispecific ADCs using Adagene’s proprietary technology— a big step forward in advancing safer, more targeted cancer therapies. investor.adagene.com/news-releases/… #Adagene #ADC #CancerTherapy #Biotech #SAFEbody

We are thrilled to announce the expansion of our partnership with @sanofi, highlighting the potential of our SAFEbody platform and the clinical proof of concept for ADG126, our masked anti-CTLA-4 program.Learn more: loom.ly/5lPD5Gw #CTLA4 #ColorectalCancer

We announced updated data being presented at #ASCO25 from our Phase 1b/2 study in advanced microsatellite stable #colorectalcancer, with muzastotug (ADG126) showing a 29% confirmed ORR when added to pembrolizumab (KEYTRUDA®). Learn more:investor.adagene.com/news-releases/…

Don't miss Dr. Peter Luo, Adagene CEO, presenting "Breakthrough Immunotherapy in MSS CRC: Novel Mechanism, Modality, and Dosing Drive 33% Confirmed ORR in Phase 1b/2 Trial" at the Stifel 2025 Virtual Targeted Oncology Forum. Join live here: investor.adagene.com/news-events/ev…

Join us for a virtual KOL event on Sat, Jan. 25 at 1pm ET with Aurélien Marabelle, MD, PhD, Daneng Li, MD, and Marwan G. Fakih, MD, to discuss the current treatment landscape for patients with advanced/metastatic MSS CRC. Register: bit.ly/4jgjUUs

Honoring #AdaLovelace: a trailblazer in math & computing who inspires Adagene’s mission to redefine #cancer treatment through AI-driven antibody design. Her brilliance drives us to create safer, effective therapies like ADG126 SAFEbody, offering hope to MSS CRC patients.

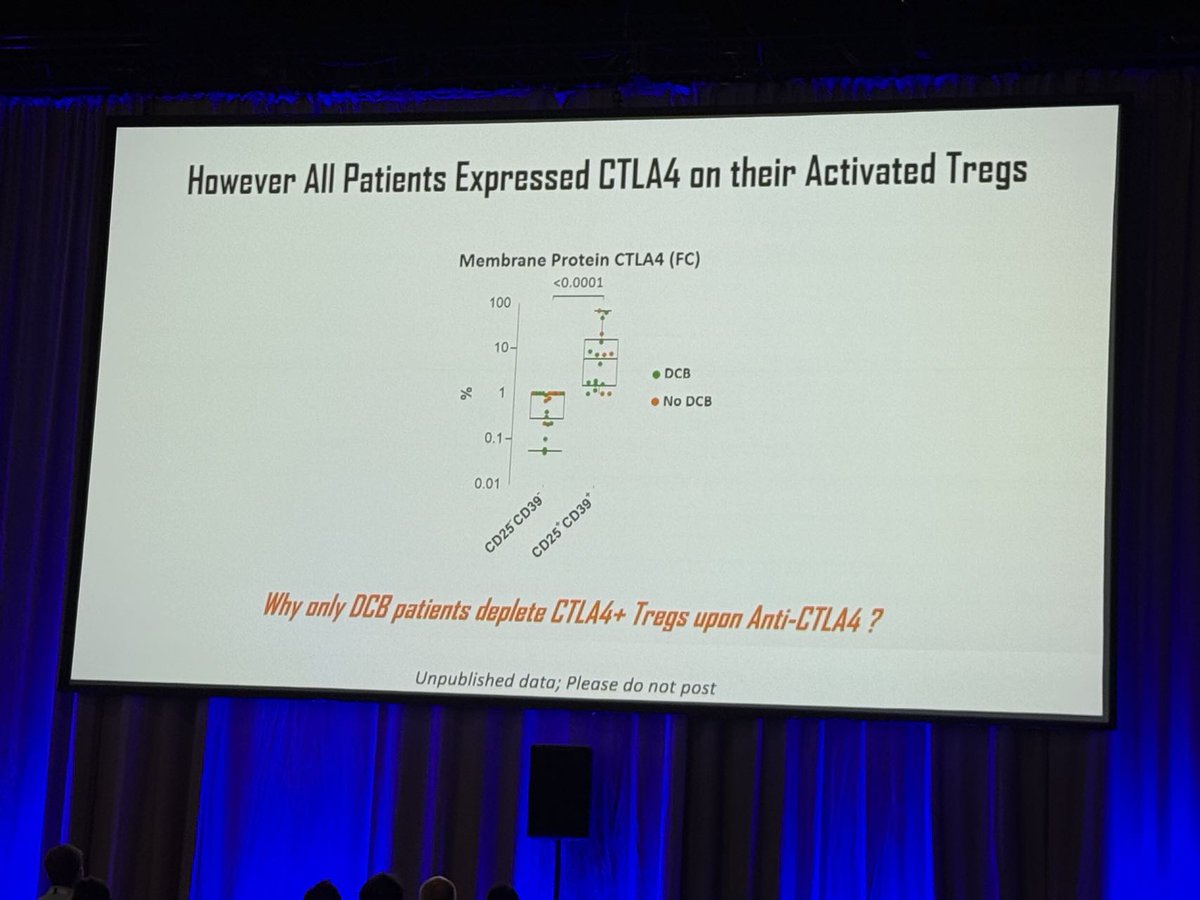
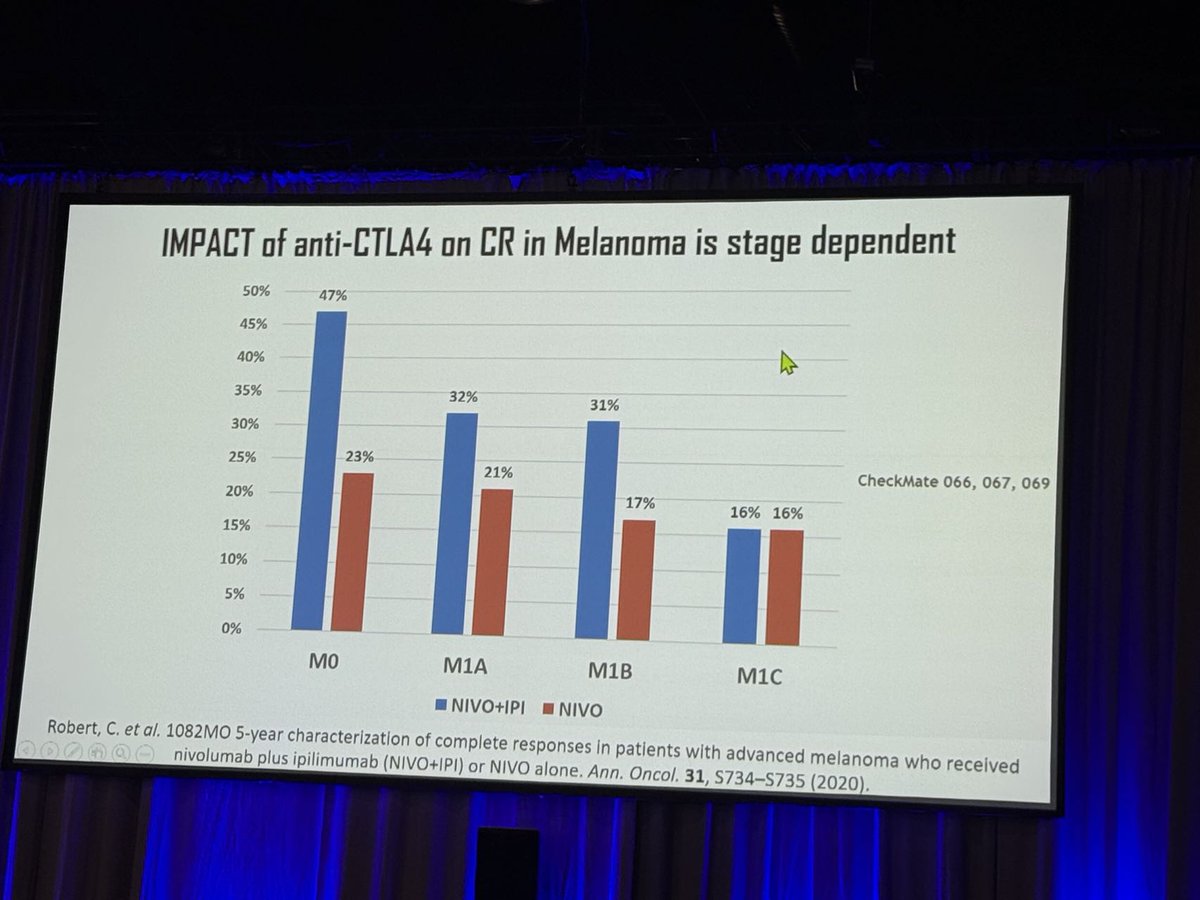
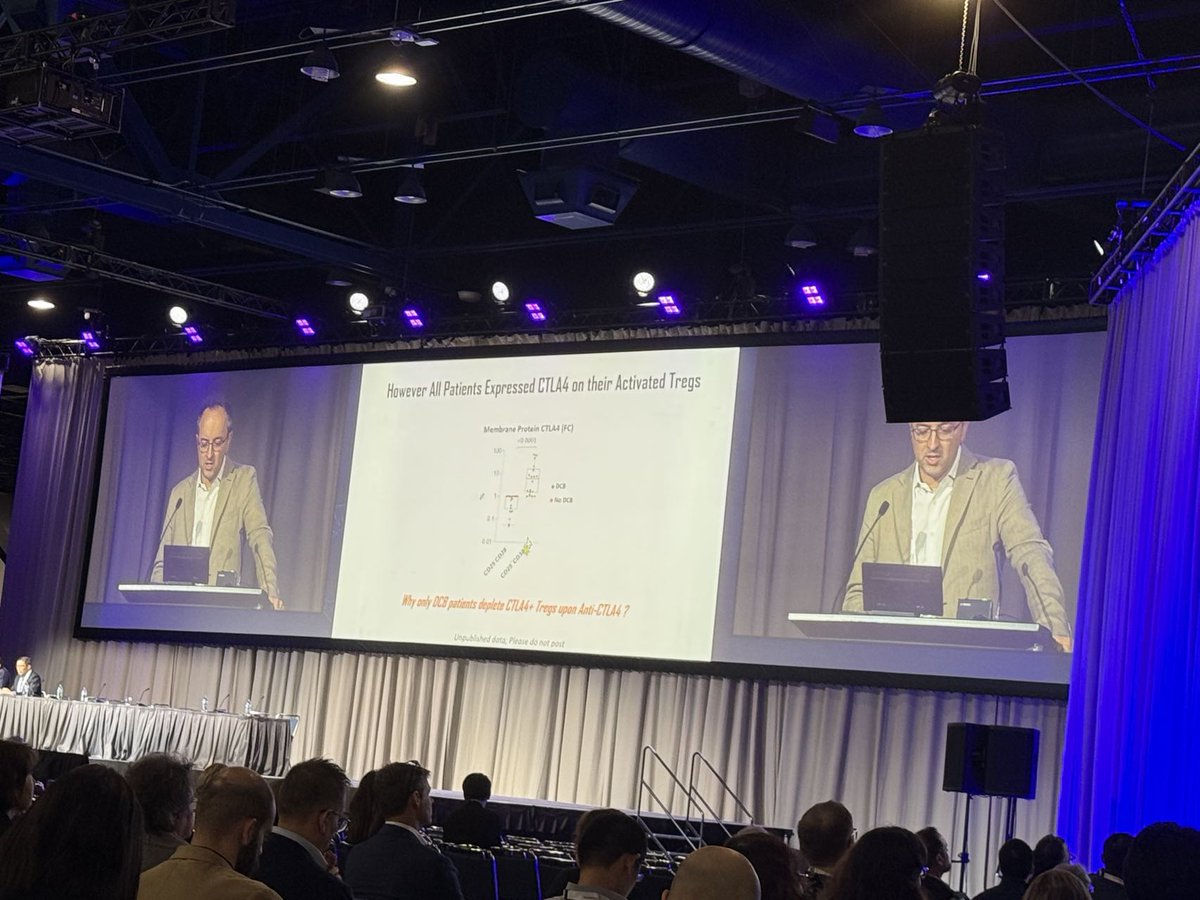
We loved Dr. Aurelien Marabelle’s talk @sitcancer 2024 on CTLA-4-mediated Treg depletion, highlighting the CTLA-4 + PD-1 combo’s benefits across cancer types. He reinforced the value of early immunotherapy & its impact on MSS CRC. Learn about ADG126: loom.ly/m_8X16s

This #Thanksgiving, we’re grateful for our global partners, advisors, trial participants, investors, and supporters who make our mission possible. To all who celebrate, we wish you joy and connection. Thank you for being part of our journey to redefine cancer treatment!

Next-gen anti-CTLA-4 therapies show promise but are limited by safety concerns & narrow therapeutic windows. Our ADG126 SAFEbody® offers a breakthrough approach, targeting CTLA-4 w/ precision, enabling treatment for MSS CRC where anti-PD-1 falls short. loom.ly/m_8X16s

Excited to showcase data on our masked anti-CTLA-4 SAFEbody, ADG126 (muzastotug) in two posters at SITC! We are proud the clinical abstract on our ongoing phase 1b/2 trial evaluating ADG126 in MSS CRC was selected by SITC as a Top 100 abstract out of 1439! loom.ly/PZ0xcVc

Every day at Adagene, we step closer to advancing cancer treatment. Our antibody development and masking platforms, including SAFEbody® ADG126, offer hope worldwide. Stay tuned as we continue to make significant progress. Learn more: loom.ly/k_8iDOw

Excited to share that our ADG126 (muzastotug) poster was selected for the Top 100 at #SITC2024! This highlights the potential of our masked anti-#CTLA-4 therapy, w/ enhanced dosing & efficacy, especially in cold/PD-L1 low tumors. Join us Nov 9 in Houston! loom.ly/YxQuPlg

Join us on Oct 12 at #SAPA2024 where our CEO, Dr. Peter Luo, will present: "Unleashing the Therapeutic Efficacy of Anti-CTLA-4 + Anti-PD-1 in MSS CRC Cold Tumor & PD-L1 Low Expression Tumors." He'll be joined by other industry leaders to discuss exciting advancements. #Oncology

Join us 10/9 for our posters at the upcoming SITC annual meeting on our lead program, ADG126 (muzastotug), a masked anti-CTLA-4 antibody. Learn how the wider therapeutic index for ADG126 can help address patient populations w/ serious unmet needs. loom.ly/YxQuPlg

With safety comparable to historical data for pembro monotherapy, our masked ADG126 in combo with pembro showed an encouraging efficacy signal, durable disease control and an early survival benefit in MSS CRC patients. loom.ly/u18V-Yk Thanks to Daneng Li!

Join us at Cantor tomorrow! Dr. Peter Luo, CEO, will present a Fireside Chat webcast @ 2:30pm ET. Following #ESMO2024, we shared exciting data showing the best-in-class potential of our anti-CTLA-4 ADG126 SAFEbody + pembro in advanced/metastatic MSS CRC. loom.ly/5Wl4nao
