
Andrew Armstrong
@AarmstrongDuke
Professor of Medicine, Surgery, Pharmacology and Cancer Biology, Duke Cancer Institute, Durham NC
Choosing an ARPI in the mHSPC setting is a highly individualized decision. Our recent MAIC analysis compares ARCHES and ARANOTE similar cohorts for efficacy of enza vs daro for improving rPFS. Interesting results! tandfonline.com/doi/full/10.10… @DukeGUCancer @AzadOncology @DukeCancer

Immune checkpoint blockade in MMRD mCRPC is effective! With some caveats… See our multicenter study results out today! @EurUrolOncol @DukeCancer @DukeGUCancer @NivenMehra @EmmanuelAntona1 sciencedirect.com/science/articl…
Breaking News from ph3 Embark trial👉Enzalutamide+ADT improve overall survival (OS) vs. ADT alone in Non-Metastatic HSPC #Prostatecancer Congrats to @SFreedlandMD @nealshore & team! Earlier enza works better! Link👉 rb.gy/iyc3f2 @OncoAlert @urotoday @PCFnews
We’re proud to share that the @US_FDA has granted Breakthrough Device Designation to ArteraAI Prostate—the first and only AI-powered risk stratification tool for prostate cancer to receive this status. A step forward for personalized, risk-based care: mpo-mag.com/breaking-news/…
🎥@AarmstrongDuke of @DukeCancer shares 5-year OS ARCHES data: enzalutamide + ADT delivers sustained survival benefits in mHSPC across disease volumes—confirming durable long-term efficacy. 📺ow.ly/UswS50W4W9c @ASCO #ASCO25 #PCSM #TrialUpdate #CTSM #UroOnc #UroSoMe
Congrats @AarmstrongDuke for the 5-yr follow-up OS results from ph3 Arches trial in mHSPC #prostatecancer with enzalutamide @ASCO #ASCO25 👉66% probability of survival at 5 yrs & 30% reduction of risk of death with enzalutamide👇@OncoAlert @urotoday @PCF_Science @AzadOncology
Thank you! #MedIQASCO25 and #ASCO25 for their support and @pfizer and @AstellasUS and @DukeGUCancer for so much support in these meaningful results!
Updated results from #ARCHES in mHSPC showing sustained benefits of #enzalutamide with minimum 5 year followup. 3 year survival benefit in high volume disease! Amazing results! @AarmstrongDuke @AzadOncology #ASCO25
Niraparib plus Abi delays rPFS in HRR+ particularly BRCA2m mHSPC in AMPLITUDE! #ASCO25 #MedIQASCO25 HR 0.63 is clinically meaningful.


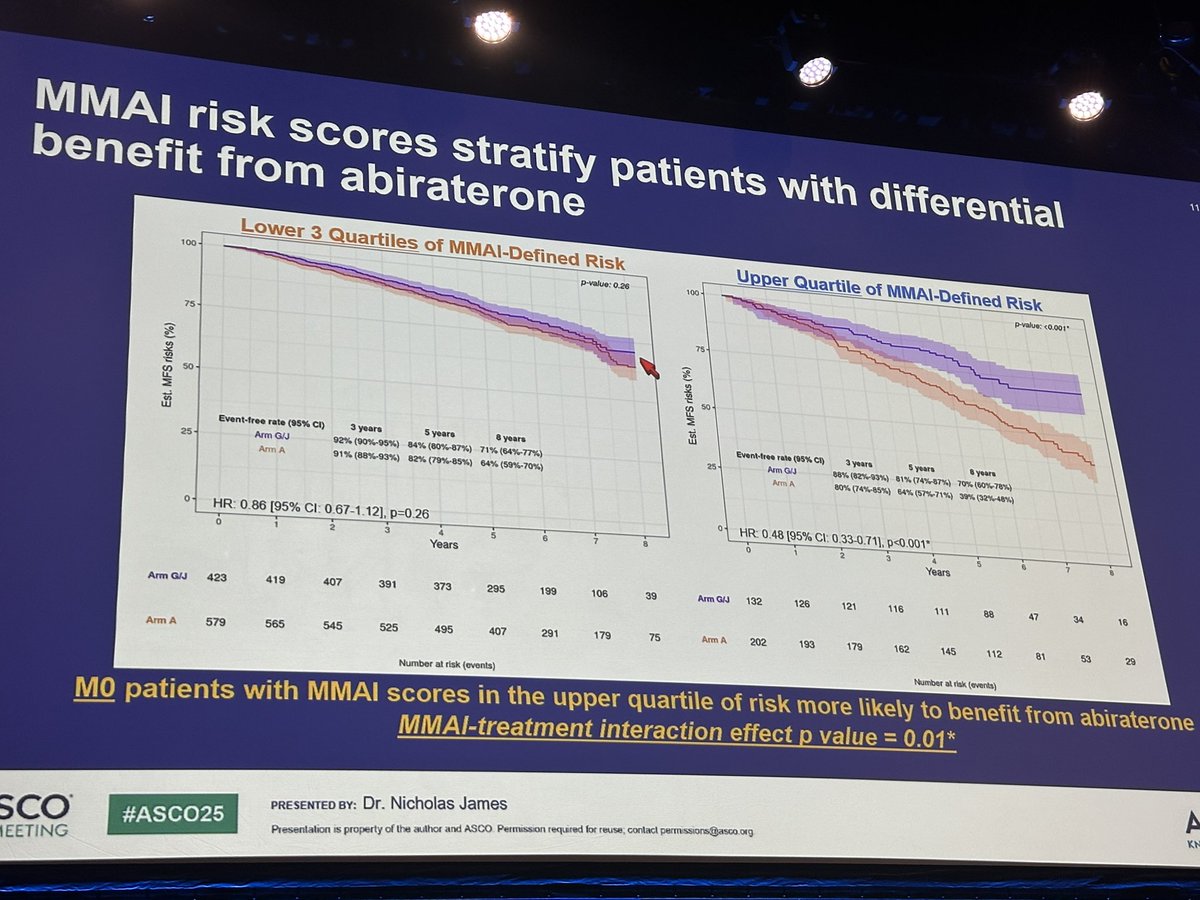
Digital pathology and clinical multimodal AI again can predict treatment needs in prostate cancer, now with abiraterone in STAMPEDE at #ASCO25 #MedIQASCO25 @arteraAI in very high risk disease! Predictive and prognostic for MFS, PCSM.

CAN-2409 is the first oncolytic and immunogenicity virus to delay progression in intermediate risk prostate cancer! Great work from @JohnsHopkins Ted DeWeese at #ASCO25 #MedIQASCO25. N=744 HR for DFS 0.52 p=.0155


Terbium-PSMA RLT has activity and safety in mCRPC, very similar to Lu177! Need more comparative data but nice to see alternative beta emitters and first Auger emitter in patients! #MedIQASCO25 #ASCO25 @PeterMacCC PSA50 of 70%, rPFS 11 mo, well tolerated.


Bullseye trial shows delays in progression in PET mHSPC without ADT. But short lived…#ASCO25 #MedIQASCO25. Is this better than MDT?

Highlighting this @ThePCCTC and @DukeGUCancer trial of a novel CTLA engaging IO plus Lu177-PSA-617 therapy in men with mCRPC at #ASCO25 #MedIQASCO25 with @TiansterZhang . Clear activity and safe!

Sneak preview of @AarmstrongDuke presenting ARCHES 5-year OS data of ENZA + ADT in pts with mHSPC on Tuesday 9:45 am Oral session, Hall D1. Probability of being alive 66%, so 2/3 of pts still alive after 5 years. 115k pts in global study. @Dukecancer @DukeGUCancer
Pasritamig is active! And hk2 is a selective target in prostate cancer. #ASCO25 abstract 5017 shows this new BiTE has an ORR of 8% with 43% PSA50 and rPFS 7.9 mo with CRS in <10%. #MedIQASCO25 worth further study @ThePCCTC @JNJNews see ascopubs.org/doi/abs/10.120…
Interesting EVOLUTION for immunoradioligand combination of ipi/nivo in mCRPC: greater responses and rPFS vs Lu177-PSMA-617 alone @PeterMacCC at #ASCO25 #MedIQASCO25 but more cardiac toxicity. Need better IOs in prostate cancer!

Targeting B7-H3 in post ARPI refractory mCRPC pays off with a new ADC: DB-1311/BNT324. ORR of 42% and 6 mo PFS rate of 68% warrants future controlled trials! Abstract 5015 at #ASCO25 #MedIQASCO25 @ThePCCTC




On the eve of #ASCO25, I am delighted to share results from the correlative analyses of the @ANZUPtrials phase II TheraP study, now published in @NatureMedicine. Massive effort supported by dedicated co-investigators, coordinators, patients & families from 11 study sites across…
Enjoyed this NPR discussion on prostate cancer with @WFAE’s Mike Collins and @unchealth urologist Kathryn Hacker Gessner. wfae.org/show/charlotte… @DukeGUCancer @DukeCancer

This is a great story of one of my ARCHES patients on @WRAL highlighting our data coming out at #ASCO25. Need to watch on your computer: wral.com/lifestyle/heal… @DukeGUCancer @AstellasUS @DukeCancer @AzadOncology
